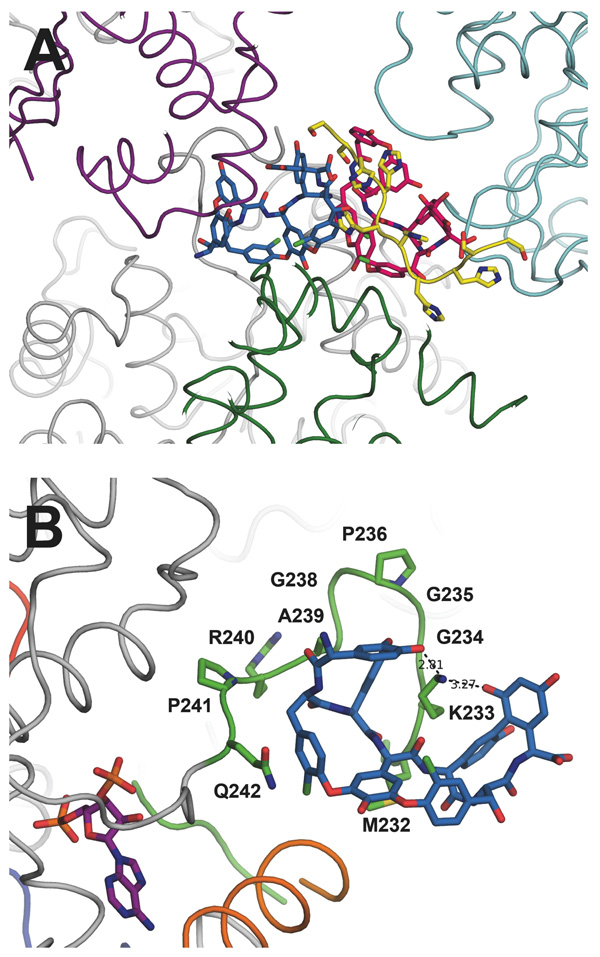
Figure 5.
(A) Two molecules of teicoplanin aglycone were modeled in the Teg12-ternary complex, colored sky blue and hot pink. Their location within the crystal appears to mediate the packing of several Teg12 monomers, as shown in purple, cyan, dark green, and grey. The two teicoplanins belong to the grey Teg12 in the crystal. Also shown in yellow is the 6X-His portion of the N-terminal derived tag from the pET28a expression vector. The tag-aglycone interaction is proposed to mimic the binding of the aglycone to its cellular target, D-Ala-D-Ala of the bacterial cell wall. (B) A close-up of the GHL loop-aglycone complex from the Teg12-ternary structure. Side chains for all alanine replacement experiments are shown. Lys233 intercalates into the glycopeeptide makes hydrogen bond contacts with residues 1 and 7 of the aglycone. For orientation purposes, the molecule of PAP is shown in purple. Also shown in orange is the GHL helix.