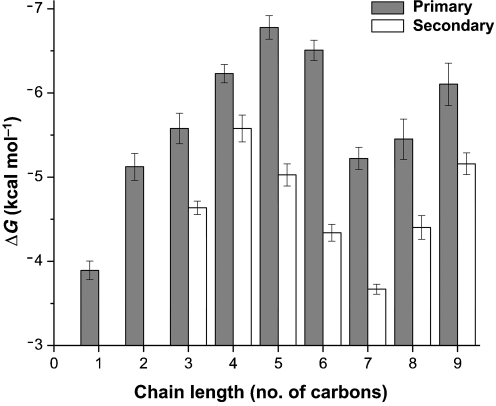
Fig. 2.
Alcohol binding energies to BMOH. Binding energies of primary alcohols (shaded bars) and secondary alcohols with the hydroxyl group at the second carbon position (white bars) were calculated from the observed competitive inhibition constants using the equation ΔG=−RT ln(Kic−1), where T is the temperature in Kelvin (298 K) and R is the universal gas constant.