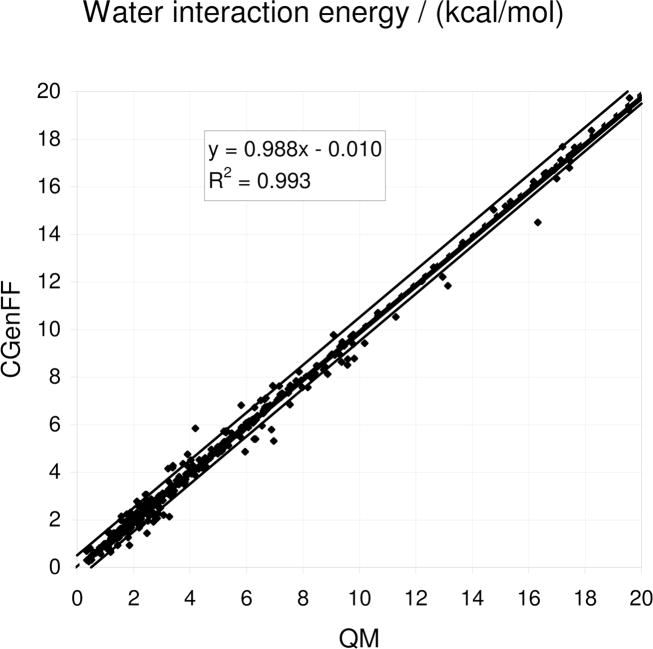
Figure 7.
Comparison of the QM and CGenFF minimum water interaction energies for the model compound-water monohydrate interactions. The QM level of theory is MP2/6–31G(d) for model compounds containing sulfur atoms and scaled HF/6–31G(d) for all remaining compounds. The green lines represent deviations of ± 0.5 kcal/mol.