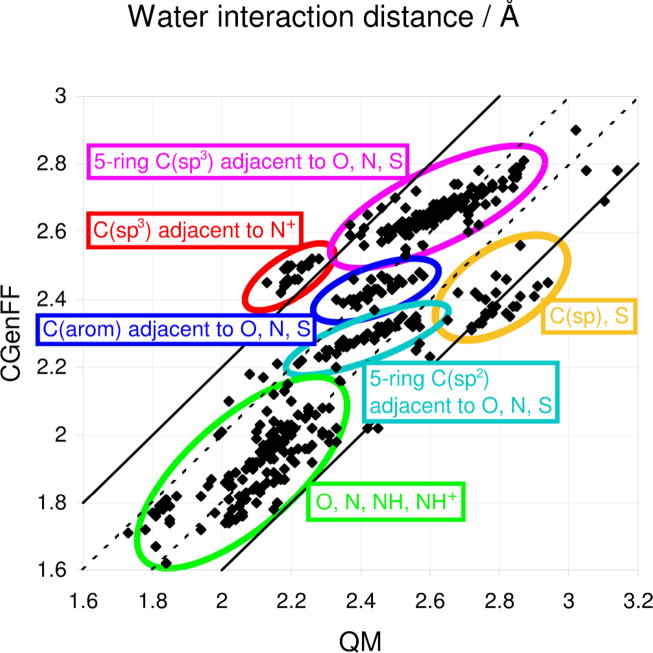
Figure 8.
Comparison of the QM and CGenFF minimum water interaction distances for the model compound-water monohydrate interactions. The QM level of theory is MP2/6–31G(d) for model compounds containing sulfur atoms and HF/6–31G(d) for all remaining compounds. Green: direct interaction with heteroatoms (O, N, NH, NH+); cyan: 5-membered ring C(sp2) adjacent to heteroatom; blue: C(aromatic) adjacent to heteroatom; magenta: 5-membered ring C(sp3) adjacent to heteroatom; red: C(sp3) adjacent to N+; orange: C(sp), S. The bottom dotted line represents the situation where the CGenFF distance is 0.2 Å smaller than the QM distance, as ideally would be the case for regular hydrogen bonds. The top dotted line represents CGenFF=QM, which is the ideal case for interactions that have a weak hydrogen bonding character. The top and bottom solid lines represent deviations of ± 0.2 Å.