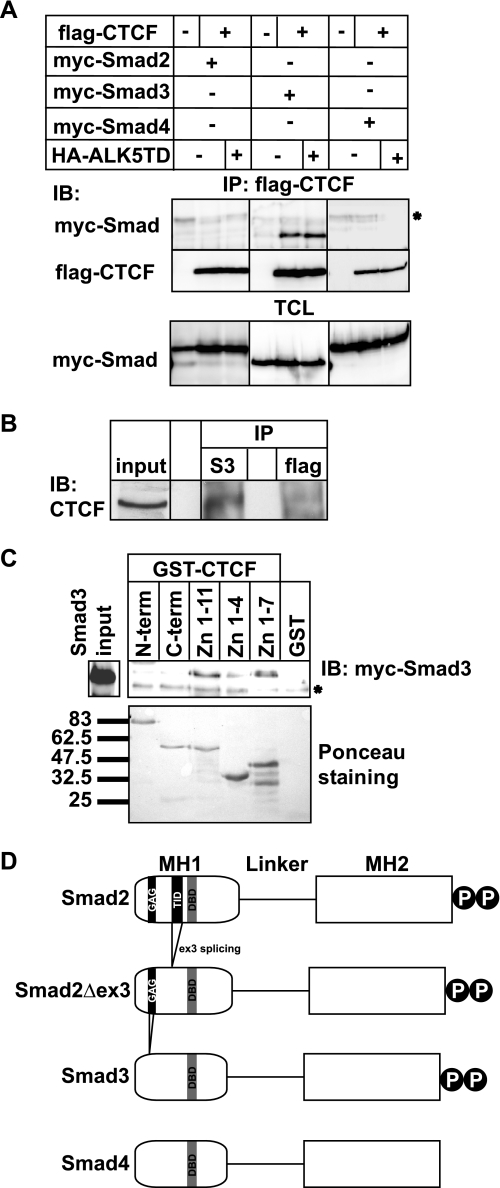
FIGURE 1.
Smad3 interacts with the zinc finger domain of CTCF. A, immunoprecipitation (IP) of FLAG-CTCF followed by immunoblotting (IB) for the indicated proteins after transfection of HEK293T cells with the indicated plasmids. Total cell lysates (TCL) demonstrate the input Smad levels. An asterisk indicates a nonspecific protein. B, co-immunoprecipitation of endogenous CTCF with endogenous Smad3 from HepG2 cells in the absence of TGFβ stimulation. Immunoblots of total cell lysates are shown as input, and flag represents immunoprecipitation with a nonspecific antibody. C, GST pulldown assay of transfected Myc-Smad3 with the indicated GST fragments of CTCF. An aliquot of transfected HEK293T cells shows the Smad3 expression used as input, and Ponceau staining shows the recombinant CTCF fragments. An asterisk indicates a nonspecific protein. Molecular size markers (in kDa) are shown in the bottom panel. D, schematic diagram of Smad2, its alternatively spliced isoform Smad2Δex3, Smad3, and Smad4. The MH1, linker, and MH2 domains are highlighted, along with the C-terminal di-serine motif that is phosphorylated by the TGFβ type I receptor. Within the MH1 domain, features of the proteins discussed in this paper are emphasized: the N-terminal Smad2-specific peptide insert GAG, the exon 3 corresponding peptide insert TID, and the juxtaposed DNA-binding domain (DBD) of all Smads. Splicing of exon 3 is indicated, and the lack of the GAG insert from the sequence of Smad3 is also shown.