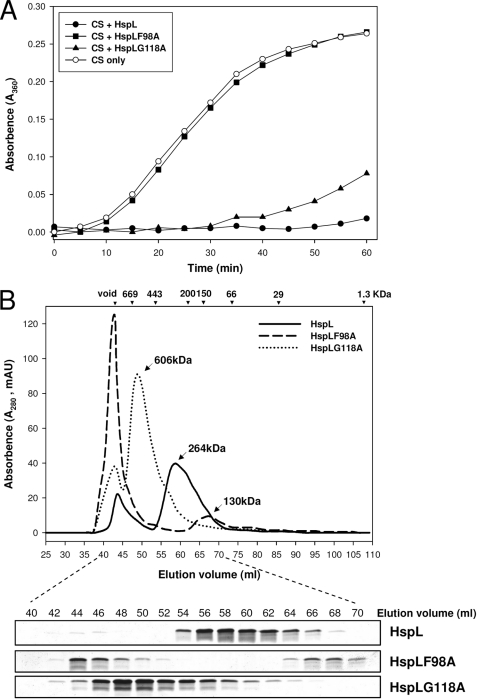
FIGURE 2.
HspL protein variants (HspLF98A and HspLG118A) were impaired in chaperone activity with altered oligomerization patterns. A, thermal aggregation protection assays using model substrate CS (600 nm) was carried out at 43 °C in the absence (○) or presence of HspL-His6 (●), HspLF98A-His6 (■), and HspLG118A-His6 (▴) at concentration of 1.2 μm. Aggregation was monitored in absorbance at 360 nm and presented as a function of time. B, 0.5 ml (1.0 mg/ml) of HspL-His6 (continuous line), HspLF98A-His6 (broken line), and HspLG118A-His6 (dotted line) were analyzed via SEC on Superdex 200 HiLoad 16/60. Blue dextran (2000 kDa) was used to determine the void volume at ∼44-ml elution volume, and calibration markers are indicated. Protein elution was monitored by absorbance at 280 nm. 10 μl of each eluted 2-ml interval was resolved in 15% glycine SDS-PAGE followed by Coomassie Blue staining.