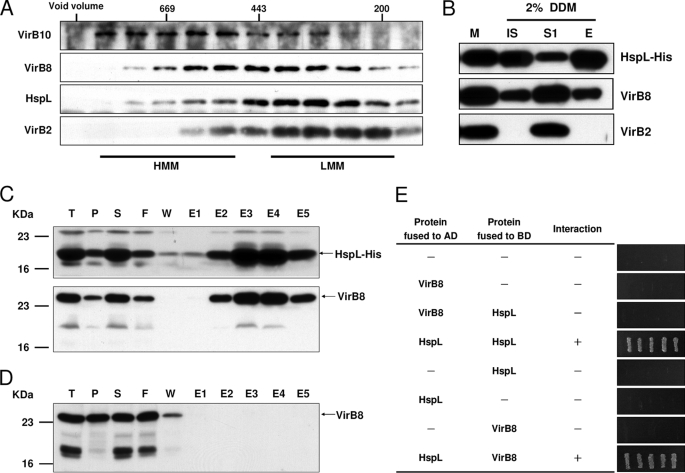
FIGURE 5.
HspL co-fractionated with VirB complexes and physically interacted with VirB8. A, membrane fractions of A. tumefaciens wild type strain NT1RE(pJK270) grown in I-medium in the presence of AS at 25 °C for 16 h were subjected for 2% DDM extraction followed by ultracentrifugation. The DDM-solubilized membrane proteins were further fractionated by SEC on Superdex 200 HiLoad 16/60, and the elution of HspL and of the T4SS components VirB10 (marker of high molecular mass core complex (HMM)), VirB2 (marker of low molecular mass pilus assembly complex (LMM)), and VirB8 (marker present in both subassemblies) was analyzed by SDS-PAGE and Western blotting with specific antisera as indicated. B, the membrane fraction of AS-induced A. tumefaciens ΔhspL(pHspL-His) strain was extracted with 2% DDM followed by ultracentrifugation to separate DDM-extracted soluble proteins (S1) from insoluble pellets (IS). The soluble fraction was incubated with Ni-NTA and eluted by elution buffer (E) and then analyzed by Western blotting detected with specific antisera as indicated. E. coli BL21(DE3) strain containing both pETHspL and pTrcB8 (C) or pTrcB8 only (D) was induced by 0.4 mm isopropyl 1-thio-β-d-galactopyranoside at 37 °C for 2 h. The soluble protein extracts were run through Ni-NTA His Bind Resins to purify HspL-His and its interacting proteins. The total protein (T), insoluble pellet (P), soluble supernatant (S), flow-through (F), wash fraction (W), and eluted fractions (E1∼E5) were analyzed by Western blot with HspL and VirB8 antisera. E, yeast two-hybrid protein-protein interaction results using combinations of various full-length HspL and VirB8 are indicated. For each transformant, five independent colonies were streaked out on synthetic dextrose medium in the absence of Ade, His, Leu, and Trp grown at 30 °C for 3 days to select the transformant expressing the interacting proteins. AD, activation domain; BD, binding domain.