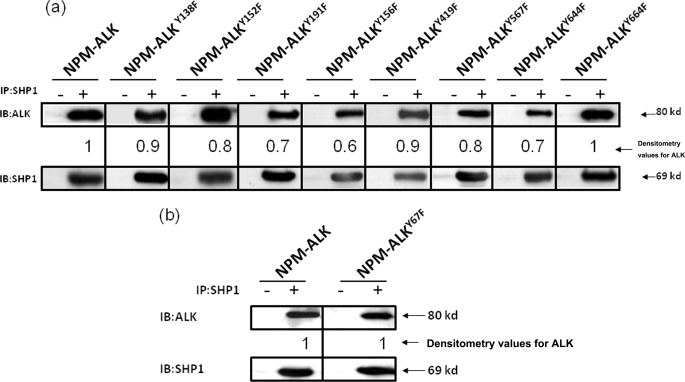
FIGURE 3.
The loss of SHP1 binding is specific for the NPM-ALKTyr-343 mutant. a, co-immunoprecipitation (IP) experiments using GP293 cells co-expressing SHP1 and 8 different NPM-ALK mutants (each of which contained a single mutation of the 8 remaining tyrosine residues known to be phosphorylated) showed no substantial or relatively minimal loss of SHP1 binding. b, a randomly selected mutant, Tyr67, which has been shown previously not to be phosphorylated, showed no loss of binding to SHP1. Results shown are representative of three independent experiments. The degree of SHP1 binding seen in each lane was assessed by densitometry, and the results were normalized to the SHP1 protein level and determined relative to the SHP1 binding to unaltered NPM-ALK. IB, immunoblot.