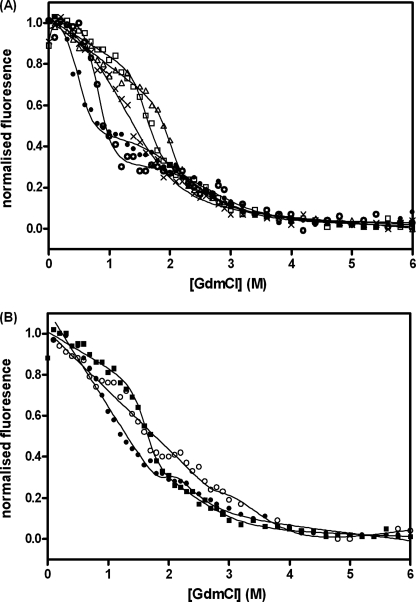
FIGURE 1.
Guanidinium hydrochloride-induced unfolding of BRCA1 BRCT domain curves. Measurements were made at 10 °C in 50 mm Hepes buffer, pH 8.5, containing 1 mm dithiothreitol. The protein concentration was 0.5 μm. The fluorescence emission at 340 nm is shown. A, wild type (□) and variants representative of the four groups (categorized according to their effects on the stability) are shown. ▵, group 1, not destabilizing, R1669Q; X, group 2, mildly destabilizing, D1692N; ○, group 3, moderately destabilizing, M1783T; and ●, group 4, very destabilizing, Y1853C. B, effect of phosphopeptide binding on BRCA1 BRCT stability. Wild type (■) and the moderately destabilizing mutant G1788D (●) are shown in the absence of peptide and for G1788D in the presence of 25 μm fluorescein-labeled phosphopeptide (○).