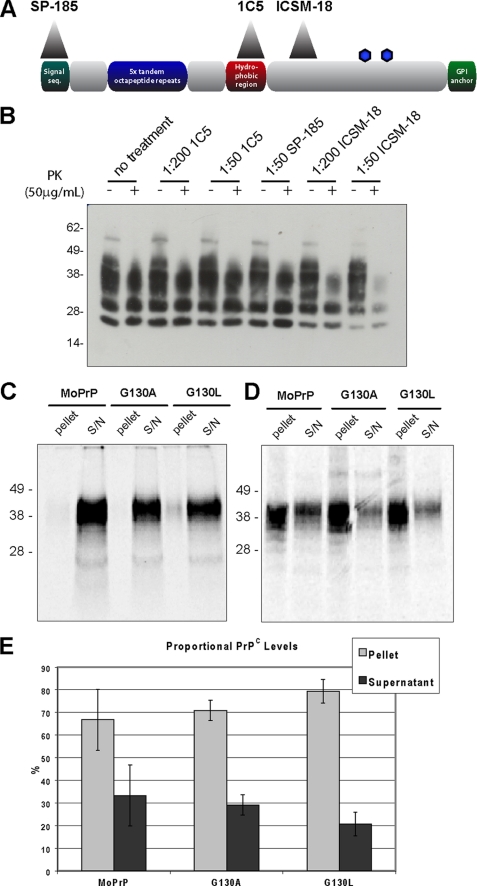
FIGURE 5.
GRR is not required for binding of PrPC to PrPSc. A, overview of PrP showing the epitopes of antibodies SP-185 (directed to PrP 1–23), 1C5 (PrP 119–130), and ICSM-18 (PrP 143–153). GPI, glycosylphosphatidylinositol; seq, sequence. B, prion-infected cells were treated with antibodies, lysed, and treated with protease to isolate PrPSc. Untreated cells display the same level of PrPSc as those treated with 1C5 or SP-185, indicating these antibodies do not cure prion infection. By contrast, ICSM-18 reduces PrPSc in a dose-dependent manner. PK, proteinase K. C, radiolabeled PrPC is mixed with PrPSc and centrifuged; binding molecules will co-precipitate into the pellet fraction. When assayed in the absence of PrPSc, MoPrP, G130A, and G130L PrPC were found to primarily reside in the supernatant (S/N) fraction. D, assaying in the presence of PrPSc leads to significant levels of MoPrP, G130A, and G130L PrPC localizing to the pellet fraction, indicating that GRR mutant PrP is capable of binding to PrPSc (n = 3). E, quantification of PrPC levels in pellet and supernatant fractions following binding assay with PrPSc demonstrates no significant difference in segregation (error bars indicate 1 S.D.).