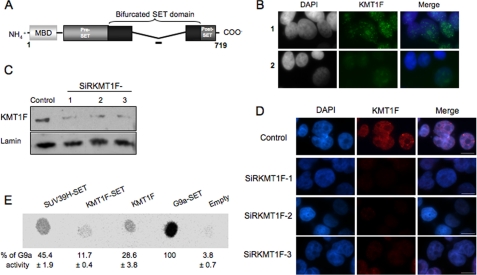
FIGURE 1.
Characterization of the KMT1F protein. A, structure of the KMT1F protein and its predicted protein domains. The KMT1F SET domain contains a central insertion and is described as a “bifurcated SET domain.” The peptide used for the production of a rabbit anti-KMT1F polyclonal antibody within a nonconserved part of the bifurcated SET domain is indicated as a bold line. B, indirect immunofluorescence in 293T cells using anti-KMT1F antibody in the presence of a control peptide (row 1) or the KMT1F-derived peptide (row 2) confirms specificity of the signal. C, the anti-KMT1F antibodies were further characterized by immunoblot on nuclear extracts of 293T cells transiently transfected with control siRNAs (Control) or three distinct small interfering RNA against KMT1F (SiRKMT1F-1, -2, or -3). The level of endogenous KMT1F protein is significantly decreased after transfection of the different siRNAs. D, transfection of the KMT1F siRNAs decreases the intensity of KMT1F staining compared with a control siRNA. Control 1 contained interphase nuclei. E, we investigated the catalytic activity of the KMT1F protein. Therefore, SET domains of SUV39H1, KMT1F and G9a, and full-length KMT1F fused to a FLAG immunoprecipitated from 293T cells were incubated in the presence of S-adenosyl-[methyl-14C]-l-methionine as a donor of methyl groups and a lysine 9-dimethylated H3 peptide (NH2-ARTKQTARK(2Me)STGGKAPRKQLC-COOH). Reaction mixtures were spotted onto an Immobilon membrane and exposed to a PhosphorImager screen, and the spots were quantified using the ImageQuant software. The percentage of signal was determined by comparing the intensity of each spot to G9A. The results from three different experiments ±S.D. are indicated. KMT1F can transfer methyl groups to lysine 9-dimethylated H3 peptide. No significant activity was observed with unmodified or H3-K9me H3 peptides.