FIGURE 3.
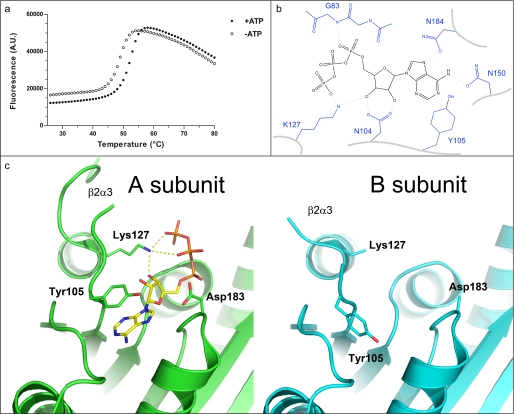
ATP binding pocket of UBA5. a, thermal stabilization by ATP. Solid and empty circles represent UBA5 protein in the presence and absence of 2 mm ATP, respectively. An increase in fluorescence is indicative of protein denaturation. Plots of fluorescence intensity versus temperature were fitted from the inflection point of the curves to interpolate the temperature at which 50% of the protein was unfolded. This transition temperature was increased by 3.2 °C in the presence of ATP. b, schematic diagram of hydrogen bonding network around ATP. ATP is shown in black, labeled side chains of UBA5 are shown in blue, and hydrogen bonds are shown as dashed lines. c, the ATP-binding active site of the A (green) and B (cyan) subunits are shown from the same perspective, side by side. Side chains that show structural variation in the two subunits and ATP are shown in stick format. Distances between Lys127 and ATP γ- and β-phosphates and ribose are 2.7, 3.5, and 2.7 Å, respectively. A. U., absorbance units.