FIGURE 2.
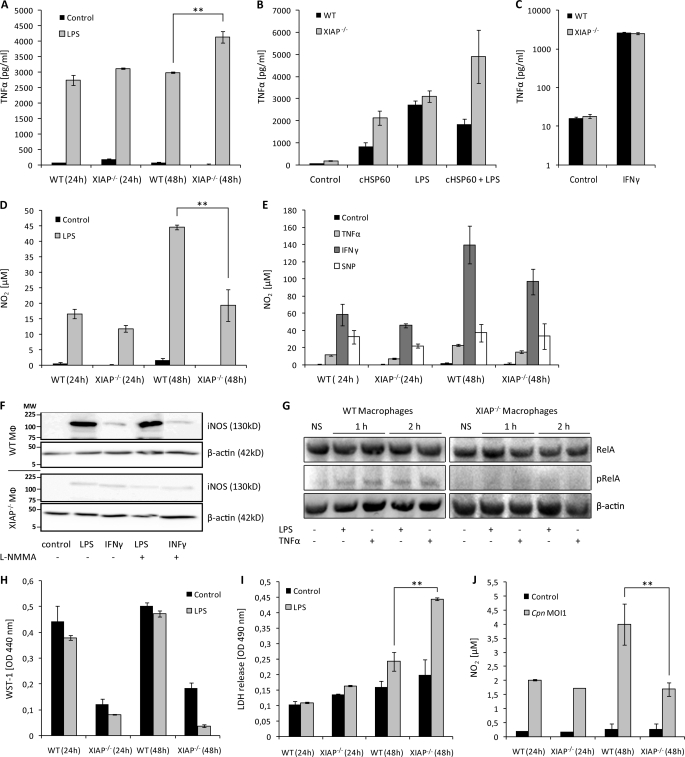
Dysregulated NO and TNF-α response in XIAP KO macrophages and mice. A, sensitization of XIAP KO macrophages for LPS-mediated TNF-α production is shown. Macrophages from WT and KO mice were cultured and treated with LPS. TNF-α concentrations were determined in culture supernatants at different post-treatment time points. Data are presented as the mean ± S.E. from three independent experiments. B, 106 peritoneal macrophages from WT and XIAP KO mice were stimulated with LPS and purified cHSP60 both separately and together. TNF-α generation was quantified at 24 h post-treatment in macrophage culture supernatant by enzyme-linked immunosorbent assay. C, XIAP is not involved in IFN-γ induced TNF-α production in macrophages. 104 peritoneal macrophages from WT and XIAP KO mice were stimulated with IFN-γ, and TNF-α generation was determined in culture supernatants at 24 h post-infection. Data represent the mean ± S.E. from three independent experiments. D, shown is poor induction of LPS-mediated NO2 in the XIAP KO macrophages. The isolated peritoneal macrophages from WT and XIAP KO mice were stimulated with LPS, and NO2 was determined in the culture supernatants at different post-treatment time points. Data represent the mean ± S.E. from three independent experiments. E, procedures were the same as in D, but macrophages were treated with inflammatory cytokines. NO2 was determined at 24 and 48 h post-treatment. Data represent the mean ± S.E. from three independent experiments. F, XIAP is required for induction of iNOS by LPS. Cells were incubated with LPS, IFN-γ, or left untreated (control), and iNOS expression was monitored by Western blot using anti- iNOS antibody (BD Pharmingen). β-Actin was detected as the loading control. Shown is one representative blot from three independent experiments with similar results. Lines indicate molecular weight markers (MW) in kDa. G, reduced stimulation of NF-κB RelA/p65 in pulmonary macrophages of XIAP KO mice is shown. CD11b+ pulmonary macrophages were purified from the lungs of WT and XIAP KO mice as described under “Experimental Procedures.” Purified macrophages were either left untreated (NS) or were treated for 1 or 2 h with TNF-α or LPS as indicated. Macrophages were lysed in radioimmune precipitation assay buffer and subjected to SDS-PAGE and Western blotting. RelA/p65 and phospho-RelA/p65 (Ser(P)-536) were detected as described under “Experimental Procedures.” Note that because of the reduced expression of RelA/p65 in XIAP KO macrophages (Fig. 1E), loading was adjusted to achieve similar levels of RelA/p65 in samples derived from WT and XIAP KO macrophages. H, poor stimulation of XIAP KO macrophages is shown. Both WT and XIAP KO CD11b+ peritoneal macrophages were treated with LPS, and metabolic activity was measured at the indicated time intervals. Data present mean of optical density (OD) ± S.E. from three independent experiments. I, shown is activation induced death of XIAP KO macrophages. The supernatants from the macrophages treated as in H were collected, and cytotoxicity of LPS treatment was detected by lactate dehydrogenase release at 490 nm in a spectrophotometer. Data are represented as the mean of lactate dehydrogenase release ± S.E. from three independent repeats. J, shown is reduced NO2 generation in XIAP KO-infected macrophages. WT and XIAP KO Mac-1+ macrophages (1 × 104) were infected with C. pneumoniae at multiplicity of infection 1(Cpn MOI1), and infection-induced NO2 was measured at indicated time intervals post-infection. Data present the concentration of NO2 generated ±S.E. from three independent experiments. **, p ≤ 0.01.