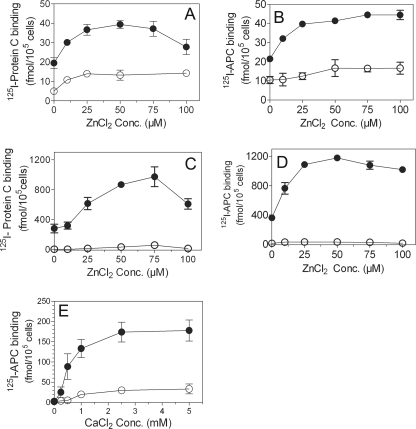
FIGURE 1.
Zinc ions enhance Ca2+-mediated protein C and activated protein C binding to endothelial cell protein C receptor. Confluent monolayers of HUVEC (A and B) or CHO-EPCR cells (C and D) cultured in 24-well plates were treated with control vehicle or EPCR blocking mAb (10 μg/ml) for 30 min at room temperature. The cells were then chilled and incubated with 125I-protein C (80 nm, A and C) or 125I-APC (80 nm, B and D) and varying concentrations (0–100 μm) of ZnCl2 in the presence (●) or absence (○) of CaCl2 (5 mm) for 3 h at 4 °C. After the 3-h incubation, the supernatants were removed, the cells were washed, the bound 125I-protein C or 125I-APC was eluted with 0.1 m glycine, and the radioactivity in the eluate was counted. The data shown in the graph represent EPCR-specific binding, which was determined by subtracting the nonspecific binding (binding observed with cells pretreated with EPCR blocking mAb) from the total binding (binding observed with cells pretreated with control vehicle). E, CHO-EPCR cells, pretreated with control vehicle or EPCR blocking mAb, were incubated with 125I-APC (10 nm) and varying concentrations of CaCl2 in the presence (●) or absence (○) of 25 μm Zn2+. After 3 h of incubation at 4 °C, 125I-APC bound to EPCR was determined as described above. Conc., concentration.