Abstract
The concept of high-resolution manometry (HRM) is to use sufficient pressure sensors such that intraluminal pressure can be monitored as a continuum along luminal length much as time is viewed as a continuum in conventional manometry. When HRM is coupled with pressure topography plots, pressure amplitude is transformed into spectral colors with isobaric conditions indicated by same-colored regions on the display. Together, these technologies are called high-resolution esophageal pressure topography (HREPT). HREPT has several advantages compared with conventional manometry, the technology that it was designed to replace. (i) The contractility of the entire esophagus can be viewed simultaneously in a uniform format, (ii) standardized objective metrics can be systematically applied for interpretation, and (iii) topographic patterns of contractility are more easily recognized and have greater reproducibility than with conventional manometry. Compared with conventional manometry, HREPT has improved sensitivity for detecting achalasia, largely due to the objectivity and accuracy with which it identifies impaired esophagogastric junction (EGJ) relaxation. In addition, it has led to the subcategorization of achalasia into three clinically relevant subtypes based on the contractile function of the esophageal body: classic achalasia, achalasia with esophageal compression, and spastic achalasia. Headway has also been made in understanding hypercontractile conditions, including diffuse esophageal spasm and a newly described entity, spastic nutcracker. Ultimately, clinical experience will be the judge, but it seems likely that HREPT data, along with its well-defined functional implications, will improve the clinical management of esophageal motility disorders.
INTRODUCTION
New technology has a way of making the old seem quaint. Witness the evolution from typewriters to word processors; the purpose may be similar, but who would ever use a typewriter today? Its not stretching the point too much to suggest that a similar leap has occurred in the realm of esophageal function testing. Perfusion manometry with three to eight pressure sensors, viewed as a series of pressure graphs, was a major development in 1970s and 1980s, but now seems primitive compared with solid-state high-resolution esophageal pressure topography (HREPT) plots that have moved to the forefront. True, the diagnostic objectives are largely the same, however, what I hope to convey herein is how HREPT has improved the accuracy and reproducibility of diagnostics in the field of esophageal motor disorders.
WHAT IS HREPT?
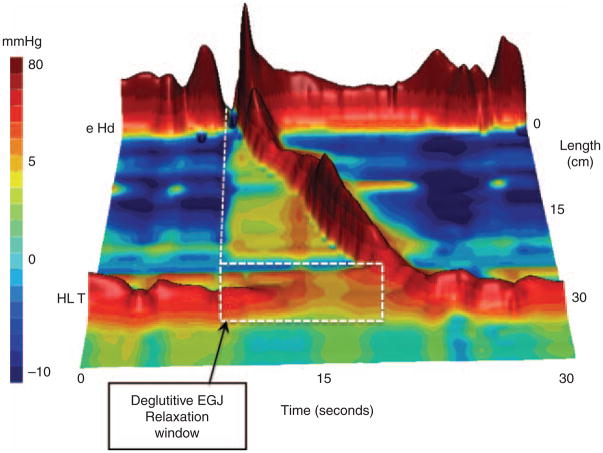
First of all, HREPT is not synonymous with high-resolution manometry (HRM). HRM simply implies that manometry is performed with a multitude of closely spaced pressure sensors; by convention, this means 1 cm or less spacing between sensors based on the observation that with 1 cm spacing there is negligible loss of pressure data between sensors (1). Pressure topography, on the other hand, is a method for viewing and analyzing manometric data. Alternatively called isobaric contour plots or, in recognition of the individual who first pioneered these, Clouse Plots (1,2), these plots lay out a coordinate system of time on the x axis, sensor position on the y axis, and then localized pressure values represented by color, three-dimensional elevation, or both (Figure 1) within that coordinate system. Hence, although not synonymous, HRM is a prerequisite for HREPT. One can construct pressure topography plots out of manometry data with sensors spaced two or more cm apart, but the validity of doing so is questionable because interpolating the intermediate values between two widely spaced pressure sensors assumes that a smooth pressure continuum exists between them and this simply may not be the case. The obvious example would be a sphincter with a manometric sensor above and below it; interpolating between the two sensors would fail to detect the sphincter at all. Only with closely spaced pressure sensors can this pitfall be avoided.
Figure 1.
Typical swallow pressure topography spanning from the pharynx to stomach of a normal subject with normal peristalsis and normal EGJ relaxation imaged as a landscape plot with elevation proportional to the magnitude of intraluminal pressure. As such, the peristaltic contraction appears as a ridge progressing from the upper sphincter to the EGJ over a span of about 10 s. Crucial in the interpretation of pressure topography plots is the assessment of deglutitive EGJ relaxation. This calculation is made within the highlighted rectangular area spanning across the EGJ from the time of the swallow to the arrival of the peristaltic contraction by the method summarized in Figure 2. EGJ, esophagogastric junction.
To summarize the above discussion, HREPT is a new technology and format for collecting and displaying manometric data. It was not intended to be a complimentary technology to conventional manometry as in the case with intraluminal impedance monitoring; it is a replacement technology. Consequently, when evaluating the utility of HREPT as a clinical study, it makes little sense to compare it with intraluminal impedance monitoring. Rather, the logical comparator is conventional esophageal manometry. Compared with conventional esophageal manometry, HREPT has several distinct advantages: (i) it is easier and quicker to perform high-quality studies of uniform format because proper probe placement is visually obvious and, once achieved, it is not necessary to reposition the probe for the duration of the study, (ii) the technique lends itself to the development of standardized objective measures of peristaltic and sphincter function, and (iii) the process of interpretation is more intuitive and more easily learned by trainees or practitioners naive to esophageal manometry in any format (3).
Accepting the premises that HREPT can precisely quantify the contractility of the esophagus and its sphincters and that it was designed to replace conventional esophageal manometry in clinical practice, it is reasonable to ask how it compares and what it adds to conventional manometry. These are important questions that can be addressed on a purely technical level or, alternatively, from a very practical clinical vantage point. As this discourse in on the impact of HREPT on clinical practice, lets focus on the impact of HREPT on two of the most common indications of esophageal manometry: (i) the evaluation of suspected achalasia and (ii) the evaluation of suspected diffuse esophageal spasm (DES).
HREPT VS. CONVENTIONAL MANOMETRY IN THE DIAGNOSIS OF ACHALASIA
Achalasia is not only the best-defined esophageal motor disorder, but also the one with the most specific treatment. According to current conventional manometry classification, the criteria for diagnosing achalasia are incomplete lower esophageal sphincter (LES) relaxation (>8mmHg nadir) and absent peristalsis (4). In addition, three subgroups of atypical disorders of LES relaxation are recognized: (i) instances with some preserved peristalsis, (ii) instances of esophageal contractions with amplitudes >40mmHg, and (iii) instances of complete LES relaxation but of inadequate duration. The second group is sometimes referred to as “vigorous achalasia” but that terminology was discouraged by the authors of the conventional classification because no consensus existed as to whether or not such subclassification was meaningful. Notable deficiencies in this classification scheme are as follows: (i) there is no rigorously defined methodology for assessing either LES relaxation or esophageal body function, (ii) there is no stipulation for the required duration of LES relaxation, and (iii) there is no distinction between pressure waves within the esophageal body attributable to intrabolus pressure, spasm, or peristalsis. Each of these limitations can be systematically addressed with HREPT.
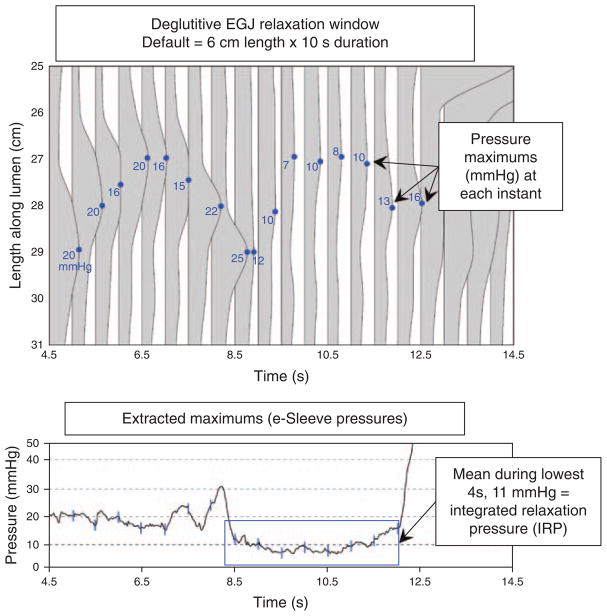
Several nuances of quantifying LES relaxation emerge in HREPT (5,6). First of all, the crural diaphragm is superimposed on the LES and swallowing does not inhibit the crural diaphragm. Consequently, other than in instances of hiatal hernia, one is actually measuring esophagogastric junction (EGJ) relaxation, not LES relaxation. Second, the sphincter moves an average of 2 cm proximally during swallowing as a consequence of esophageal shortening with longitudinal muscle contraction; in extreme instances this can be as much as 9 cm. Consequently, a point sensor positioned within the sphincter before swallowing can be distal to the sphincter during relaxation and subject to the artifact of “pseudorelaxation” (6). Third, it is overly simplistic to think of EGJ relaxation pressure as solely indicative of LES relaxation. Actually, at any one instant the measured pressure is the greatest of three possible contributions: LES pressure, crural diaphragm contraction, or intrabolus pressure as the swallowed water traverses the EGJ (7). For all of these reasons, the metric of nadir LES pressure as applied in conventional manometry is very insensitive for the detection of abnormal deglutitive EGJ relaxation. Hence, the development of the HREPT EGJ relaxation metric of the integrated relaxation pressure (IRP) (6). The IRP is measured within the deglutitive window capturing the axial movement of the LES attributable to longitudinal muscle contraction and spanning from the time of initiation of the swallow until the arrival of the peristaltic contraction (or 10 s in the absence of peristalsis). Thus, the IRP is similar to measuring EGJ relaxation with a Dent sleeve with the added stipulation that the relaxation pressure being reported is the average value persisting for a period of 4 s after the swallow (Figure 2). Table 1 illustrates the added yield of the IRP compared with the nadir LES or EGJ pressure in the detection of impaired EGJ relaxation in a series of well-defined achalasia patients. This improvement is of great significance because failing to detect impaired EGJ relaxation in these patients has the result of giving them an alternative diagnosis, most commonly misclassifying them as ineffective esophageal motility or DES (4).
Figure 2.
Detail of the deglutitive EGJ relaxation window illustrating the derivation of the integrated relaxation pressure (IRP). The top panel illustrates a series of pressure profiles across spanning the EGJ at 0.2 s intervals and the blue dot on each indicates the location and magnitude of the greatest pressure along each pressure profile. The lower panel graphs these extracted maximums (blue lines) along with the intermediate values that are not shown in the upper panel. The IRP requires persistence of EGJ relaxation for 4 s within the relaxation window, but the actual time periods that go into its calculation (blue box) can be contiguous, as in this example, or non-contiguous. The IRP was selected as the standard metric for EGJ relaxation because it best differentiated the impaired EGJ relaxation in achalasia from non-achalasic individuals. Adapted from Ghosh et al. (6). EGJ, esophagogastric junction.
Table 1.
Comparison of esophagogastric junction (EGJ) relaxation metrics in 62 cases of well-defined achalasia
| EGJ relaxation metric | Achalasia sensitivity (%) | False positives | False negatives (%) |
|---|---|---|---|
| Nadir pressure, conventional (≥7 mmHg) | 52 | 0 | 48 |
| Nadir HREPT pressure (≥10 mmHg) | 69 | 0 | 31 |
| Integrated relaxation pressure (≥15 mmHg) | 97 | 0 | 3 |
The nadir pressure in conventional terms was the lowest pressure recorded from the sensor centered in the EGJ, whereas the nadir high-resolution esophageal pressure topography (HREPT) pressure accounted for sphincter movement after the swallow, in essence a nadir sleeve pressure. Both exhibited very poor sensitivity for detecting achalasia because of the subset of achalasics with brief periods of EGJ relaxation to within the normal range. In contrast, the integrated relaxation pressure requires persistence of EGJ relaxation for 4 s, skipping over periods of crural contractions if necessary. Normal values were determined from 75 asymptomatic control subjects. Adapted from Ghosh et al. (6).
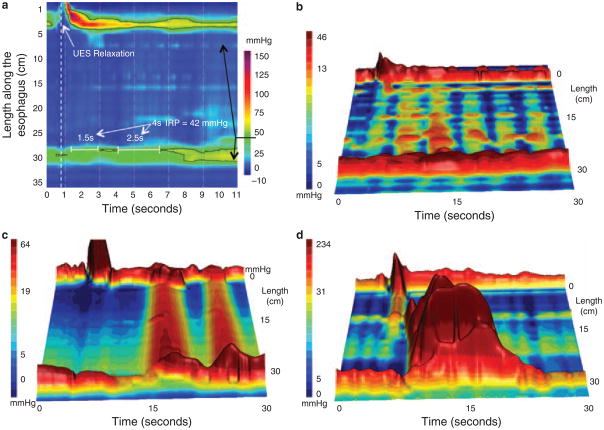
Apart from improving the sensitivity of manometry in the detection of achalasia by objectifying the definition of impaired deglutitive EGJ relaxation, HREPT has also defined a clinically relevant subclassification of achalasia based on the pattern of contractility in the esophageal body (8). A diagnosis of achalasia requires both absent peristalsis and impaired deglutitive EGJ relaxation. However, absent peristalsis is not synonymous with an absence of pressurization or contractile activity. Rather, absent peristalsis accompanying impaired EGJ relaxation can occur in the setting of esophageal dilatation with negligible pressurization within the esophagus (Figure 3a and b), pan-esophageal pressurization (Figure 3c), or with spastic contraction within the distal esophageal segment (Figure 3d). According to the Chicago Classification of HREPT, the criteria for classic achalasia are an IRP ≥15mmHg and absent peristalsis; achalasia with esophageal pressurization has an IRP ≥15mmHg and at least 20% of swallows associated with pan-esophageal pressurization to >30mmHg; and spastic achalasia has an IRP ≥15 mm Hg and a spastic contraction with ≥20% of test swallows (9). In a series of 99 consecutive patients with newly diagnosed achalasia, 21 had classic achalasia, 49 had achalasia with esophageal compression, and 29 had spastic achalasia (8). Consequently, most of the patients in that series would not be diagnosed as achalasia with conventional manometric classification. The conventional diagnosis of “vigorous achalasia,” although it never had a precise definition, would likely encompass both achalasia with esophageal compression and spastic achalasia, diagnoses with nearly opposite implications as detailed below.
Figure 3.
Achalasia subtypes are distinguished by three distinct manometric patterns of esophageal body contractility. In classic achalasia (panel a as isobaric contour plot and panel b as a landscape plot), there is no significant pressurization within the body of the esophagus and impaired EGJ relaxation (IRP of 42 mm Hg in this example). The black 42 mm Hg isobaric contour line isolates the portions of the EGJ and UES recording during which the pressure is greater than 42 mm Hg. Panel c represents a swallow from a patient the “achalasia with compression” subtype exhibiting pan-esophageal pressurization. Although high pressures may be recorded in the esophageal body in this subtype, pressure is generated by esophageal shortening in conjunction with contraction of both sphincters, rather than by spasm in the esophageal body. Radiographically, there is no esophageal retention in these patients. Panel d illustrates a pressure topography plot illustrative of spastic achalasia. The three-dimensional rendering of panel d highlights the peaks and valleys of that spastic contraction that would likely appear as a corkscrew pattern on fluoroscopy. These patients often have diffuse thickening of the distal esophageal muscularis propria. EGJ, esophagogastric junction; IRP, integrated relaxation pressure.
Ultimately, the significance of consistently identifying subtypes of achalasia is that it clarifies patient management. Preliminary data suggest this to be the case, especially in the instance of achalasia with esophageal compression, a major subtype overlooked in conventional manometric classification. Logistic regression analysis of predictors of treatment outcome in a large consecutive series of achalasia patients found pan-esophageal pressurization (Figure 3c) to be a predictor of good treatment response, whereas spastic achalasia (Figure 3d) and pretreatment esophageal dilatation were predictive of a relatively poor treatment response (8). Clearly, these nuances have not been used in previous reports of achalasia treatment outcomes. Given that the mix of achalasia subtypes within any reported series likely impacts on the outcomes in that series, this calls into question the validity of the existing treatment database in the era of HREPT. It is our suspicion that adopting these subclassifications will likely strengthen future prospective studies of achalasia management, although this clearly requires further validation.
FUNCTIONAL EGJ OBSTRUCTION: IS IT ACHALASIA?
For the reasons enumerated above, many cases that would have been labeled “atypical disorders of LES relaxation” by conventional manometric classification are recognized as achalasia with HREPT. However, even with HREPT there is still a group of patients with impaired EGJ relaxation failing to meet criteria for achalasia because they have some preserved peristalsis. Although not common, a series of 1000 consecutive patients studied with HREPT included 16 such individuals with functional EGJ obstruction exhibiting not only an IRP greater than 15 mm Hg, but also preserved peristalsis and elevated intrabolus pressure above the EGJ during peristalsis (10). The finding of elevated intrabolus pressure is important because it validates the determination of impaired EGJ relaxation; from a physiological perspective, elevated intrabolus pressure is the consequence of that impaired relaxation. Nonetheless, this is a heterogeneous group with some individuals having a variant or incomplete expression of achalasia and others likely having an undetected mechanical etiology of EGJ outflow obstruction. Consequently, it is a patient group that usually merits further evaluation with imaging studies to exclude inflammatory or malignant etiologies, be it with computerized tomography or endoscopic ultrasound, before rendering achalasia therapy.
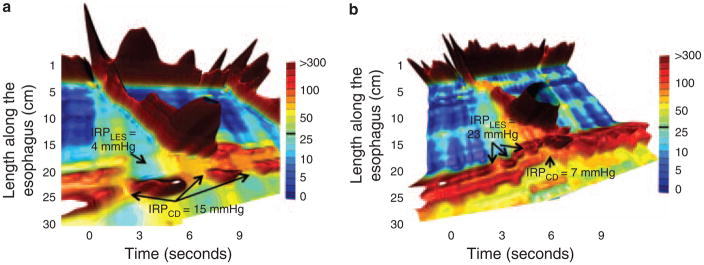
Among the 16 patients with idiopathic functional obstruction just described, 3 were noted to have hiatus hernias. In one instance, it was the crural diaphragm rather than the LES that appeared to be the focus of deglutitive resistance to bolus transit, suggesting the hernia itself to be the cause of dysphagia in this individual. A subsequent patient series specifically focused on the EGJ relaxation characteristics of patients with sliding hiatus hernia and dysphagia but without GERD symptoms by selectively restricting deglutitive relaxation measurement to the LES and crural diaphragm (11). A subset of 10 patients were found exhibiting a relative obstruction at the crural diaphragm with elevated intrabolus pressure extending through the LES to the crural diaphragm supporting the concept that sliding hiatus hernia, in and of itself, could be responsible for dysphagia (Figure 4). Consequently, patients presenting with elevated EGJ relaxation pressure in the context of a small type I hiatus hernia require careful analysis of the various components of the EGJ before making a diagnosis of achalasia and treatment with myotomy or dilation is considered.
Figure 4.
Landscape plots of two hiatus hernia patients with functional EGJ obstruction attributable to the crural diaphragm (CD) (a) or the LES (b). In each case, the corresponding IRPCD and IRPLES values are shown. Modified from Scherer et al. (14).
HREPT VS. CONVENTIONAL MANOMETRY IN THE DIAGNOSIS OF DES
Diffuse esophageal spasm is manifest clinically by episodes of dysphagia and chest pain attributable to abnormal esophageal contractions in the setting of normal LES function. Beyond that, there is little to agree upon. The pathophysiology and natural history of the disorder are ill defined. Radiographically, DES has been characterized by tertiary contractions or a “corkscrew esophagus,” but in many instances these abnormalities are actually indicative of achalasia. Manometrically, a variety of defining features have been proposed. These include uncoordinated (“spastic”) activity in the smooth muscle portion of the esophagus, spontaneous and repetitive contractions, or high amplitude and prolonged contractions. Greatest consensus exists with the concept of simultaneous contractions either with a defining minimum of 30 mm Hg or without defining amplitude (4,12). However, similar to the problems with the conventional manometric definition of achalasia, there is no distinction between pressure waves within the esophageal body attributable to intrabolus pressure, spasm, or peristalsis. Given these vagaries, it is likely that patients with a variety of disorders have been defined as DES and included in therapeutic trials of DES; not surprisingly, none such studies have demonstrated efficacy. HREPT has the potential to substantially improve upon this.
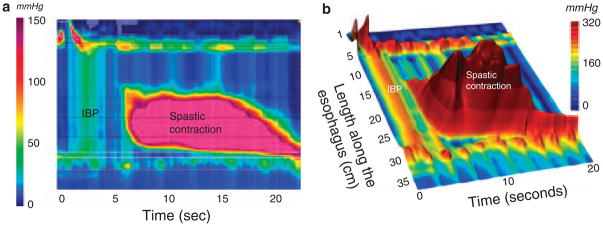
Several nuances of defining DES emerge in HREPT. First of all, there is a very important distinction to be made between a simultaneous contraction in the distal esophagus and simultaneous pressurization in the setting of impaired EGJ relaxation (Figure 5). The former fits conceptually with the conventional definition of DES, whereas the latter is simply a consequence of impaired EGJ relaxation, most commonly in the setting of achalasia. Consequently, much of what would be labeled DES on the basis of simultaneous contractions in conventional manometry is really achalasia (13). Similarly, instances of simultaneous contractions of low amplitude are almost invariably attributable to intrabolus pressure in the setting of a failed peristaltic contraction of subtle obstructive phenomenon in the distal esophagus. Consequently, instances of DES defined by the criteria of normal EGJ relaxation and a spastic contraction in the distal esophagus such as in Figure 5 are decidedly rare. So rare, that one might speculate that these are usually variant expressions of achalasia.
Figure 5.
HREPT (a) and landscape plots (b) of an extremely abnormal contraction in a patient with DES illustrating the distinction between rapidly propagated pressurization attributable to intrabolus pressure and to a spastic contraction. The distinction is especially clear in the landscape plot where the individual pressure peaks within the spastic contraction contrast with the straight ridge of intrabolus pressure (labeled IBP) bounded at each end by an area of greater pressure (the UES and crural diaphragm in this instance). This is also the distinction between pan-esophageal pressurization in Figure 3c and distal spasm in three dimensions. Despite the ridge of intrabolus pressure, this swallow has a normal IRP of 11 mm Hg. However, the contractile front velocity of the spastic contraction is very abnormal at 46 cm/s, as is the distal contractile integral (DCI) of the spastic contraction at 36,131 mm Hg•cm• •s.
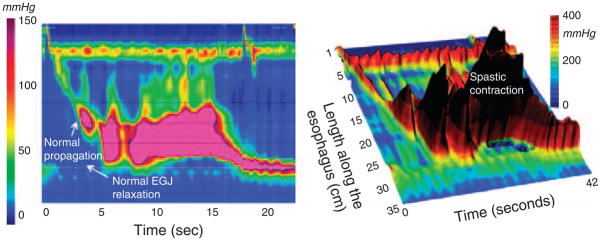
A much more consistent pattern of spasm in HREPT is of very vigorous contractions with normal deglutitive EGJ relaxation and propagation velocity. In such instances, the distal esophageal contraction can be characterized for the vigor of contraction using a newly developed measure, the distal contractile integral. The distal contractile integral integrates the length, contractile amplitude, and duration of contraction of the distal esophageal segment contraction, expressed as mmHg•s•cm (13,14). Using data from the 75 control subjects, a distal contractile integral value greater than 5000mmHg•s•cm is considered elevated. Furthermore, one particularly interesting subgroup, defined by having a higher threshold distal contractile integral (>8,000mmHg•s•cm), exhibited repetitive high amplitude contractions and was clinically discernible by the uniform association with dysphagia or chest pain (Figure 6). This “spastic nutcracker ” pattern is relatively rare, found in only three percent of a 400 patient series (14). However, it is the most consistent pattern of spasm observed in HREPT studies. From a physiological perspective the abnormality is of hyper excitability of the distal esophageal smooth muscle, establishing a clear distinction from the impaired inhibitory innervation characteristic of achalasia. Consequently, given that spastic nutcracker has a characteristic clinical presentation, unique HREPT characteristics, and a plausible defining physiological basis, it is probably the most appropriate object of future “DES” therapeutic trials.
Figure 6.
HREPT (left) and landscape plot (right) of an extremely abnormal contraction in a patient with spastic nutcracker. EGJ relaxation is normal with an IRP of 5 mm Hg. Similarly, the contraction front velocity is normal at 3.2 cm/s. However, the distal contractile integral (DCI), an indicator of the persistence and magnitude of the peristaltic contraction, is more than 10 times normal at 60,300 mm Hg•cm•s. Typical of individuals with these findings, this patient had dysphagia and chest pain.
SUMMARY
In summary, HREPT is a new methodology for assessing esophageal motility integrating HRM with pressure topography plotting. Owing to a series of technological and computational advances, HREPT represents a significant advance compared with conventional manometry, the technology that it replaces. The most obvious clinical advantage of HREPT is its improved sensitivity for detecting achalasia compared with conventional manometry. This is largely due to the objectivity and accuracy with which it identifies impaired EGJ relaxation. In addition, HREPT has led to the subcategorization of achalasia into three clinically relevant subtypes based on the contractile function of the esophageal body: classic achalasia, achalasia with esophageal compression, and spastic achalasia. Headway has also been made in understanding hypercontractile conditions, including DES. However, early clinical experience suggests that the definition of DES in conventional manometry focused on detection of simultaneous contractions is of questionable utility, usually a consequence of misdiagnosed achalasia or distal esophageal obstruction with elevated intrabolus pressure. Rather, the most consistent form of spasm observed with HREPT is in the form of a newly described entity, spastic nutcracker, characterized by normal EGJ relaxation, normal contractile front propagation, and a very abnormal peristaltic contraction characterized by high contractile amplitude and very prolonged duration. It is suggested that this entity is probably the most appropriate object of future “DES” therapeutic trials.
HREPT VS. CONVENTIONAL MANOMETRY: THE FUTURE
The era of HREPT as an esophageal function test is still relatively new and it will likely take years for a classification system of esophageal motor disorders to mature fully. However, substantial progress has occurred in the span of only a few years. An international consensus group has been formed and periodically updates the evolving process (9,15). More importantly, leading laboratories around the world have, one by one, migrated to this technology. Although all investigators and practitioners find the initial transition to be somewhat demanding, there is no going back. Again, to draw the analogy of the migration from type writers to word processors; who would ever use a typewriter today? Perhaps at the onset, the HREPT seemed just a pretty alternative to conventional manometry, but it has already improved the accuracy and reproducibility of diagnostics in the field of esophageal motor disorders. And in the future, those familiar squiggly lines of conventional manometric recordings will seem quaint indeed.
Acknowledgments
Financial support: This study was supported by R01 DK56033 from the Public Health Service.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Peter J. Kahrilas, MD.
Specific author contributions: Conceived and authored in its entirety.
Potential competing interests: None.
References
- 1.Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol. 1991;261:G677–84. doi: 10.1152/ajpgi.1991.261.4.G677. [DOI] [PubMed] [Google Scholar]
- 2.Clouse RE, Staiano A, Alrakawi A, et al. Application of topographical methods to clinical esophageal manometry. Am J Gastroenterol. 2000;95:2720–30. doi: 10.1111/j.1572-0241.2000.03178.x. [DOI] [PubMed] [Google Scholar]
- 3.Grubel C, Hiscock R, Hebbard G. Value of spatiotemporal representation of manometric data. Clin Gastroenterol Hepatol. 2008;6:525–30. doi: 10.1016/j.cgh.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49:145–51. doi: 10.1136/gut.49.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandolfino JE, Ghosh SK, Zhang Q, et al. Quantifying EGJ morphology and relaxation with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol. 2006;290:G1033–40. doi: 10.1152/ajpgi.00444.2005. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh SK, Pandolfino JE, Rice J, et al. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol. 2007;293:G878–85. doi: 10.1152/ajpgi.00252.2007. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh SK, Kahrilas PJ, Lodhia N, et al. Utilizing intraluminal pressure differences to predict esophageal bolus flow dynamics. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1023–8. doi: 10.1152/ajpgi.00384.2007. [DOI] [PubMed] [Google Scholar]
- 8.Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: a new clinically relevant classification by high resolution manometry. Gastroenterology. 2008;135:1526–33. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandolfino JE, Fox MR, Bredenoord AJ, et al. High resolution manometry in clinical practice: utilizing pressure topography to classify oesophageal motility disorders. Neurogastroenterol Mot. 2009;21:796–806. doi: 10.1111/j.1365-2982.2009.01311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scherer JR, Kwiatek MA, Soper NJ, et al. Functional esophagogastric junction obstruction with intact peristalsis: a heterogeneous syndrome sometimes akin to achalasia. J Gastrointest Surg. 2009;13:2219–25. doi: 10.1007/s11605-009-0975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandolfino JE, Kwiatek MA, Ho K, et al. Unique features of esophagogastric pressure topography in hiatus hernia patients with dysphagia. Surgery. 2009;147:57–64. doi: 10.1016/j.surg.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richter JE, Castell DO. Diffuse esophageal spasm: a reappraisal. Ann Intern Med. 1984;100:242–5. doi: 10.7326/0003-4819-100-2-242. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh SK, Pandolfino JE, Zhang Q, et al. Quantifying esophageal peristalsis with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol. 2006;290:G988–97. doi: 10.1152/ajpgi.00510.2005. [DOI] [PubMed] [Google Scholar]
- 14.Pandolfino JE, Ghosh SK, Rice J, et al. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103:27–37. doi: 10.1111/j.1572-0241.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 15.Kahrilas PJ, Ghosh SK, Pandolfino JE. Esophageal motility disorders in terms of pressure topography: the Chicago classification. J Clin Gastroenterol. 2008;42:627–35. doi: 10.1097/MCG.0b013e31815ea291. [DOI] [PMC free article] [PubMed] [Google Scholar]