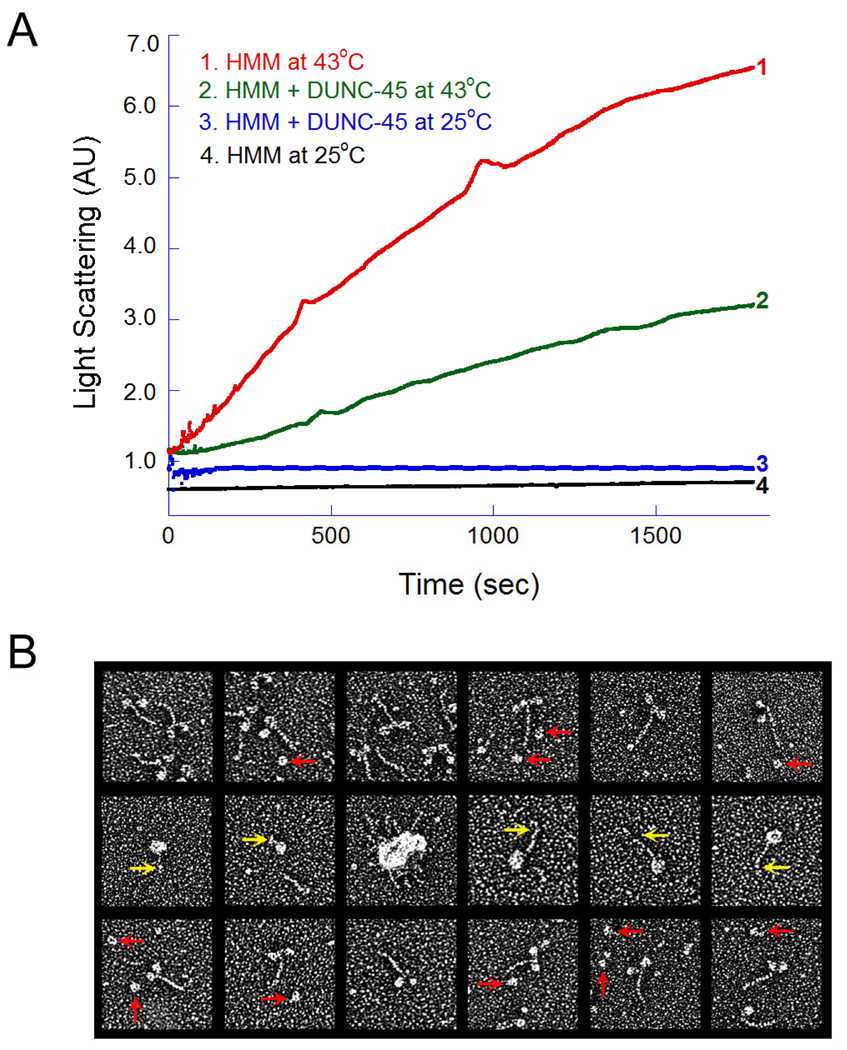
Fig. 3.
Light scattering and electron microscopy of HMM in the absence or presence of DUNC-45. (A). LS of chicken skeletal muscle myosin HMM fragment in the absence (curve 1) or presence of (curve 2) of DUNC-45. Curves 3 and 4 represent LS of HMM at 25°C in the presence or absence of DUNC-45. (B) Electron microscopy of HMM, in the absence and in the presence of DUNC-45. When HMM was incubated with DUNC-45 at 25°C the majority of the HMM molecules were well-preserved, two-headed, short-tailed structures (top row). DUNC-45 consistently appeared as a globular protein about the size of a single myosin S-1 head (red arrows, top row). When HMM was heated to 43°C for 30 min, most of the molecules collapsed and fused together to form intra- or inter-molecular aggregates through extensive head domain associations (middle row). Additionally, the short S-2 tail segment was often kinked or coiled and was not well resolved from the fused heads (middle, yellow arrows). In the presence of DUNC-45, the amount of HMM molecules forming heat-induced aggregates was dramatically reduced (bottom row).