Abstract
The specificity of binding of wheat germ ribosomes to mRNA was greatly altered by cleavage of the message. Fragmentation of reovirus mRNA allowed wheat germ ribosomes to bind and protect a variety of internal sequences which were not accessible to ribosomes in the intact message. In experiments using the polycistronic mRNA from bacteriophage R17, wheat germ ribosomes bound preferentially at the beginning of the lysis peptide and synthetase cistrons, and at a third site which may be derived from the C-terminal region of the A protein cistron. This result is similar to that reported previously in a mammalian translational system (J.F. Atkins et al., Cell 18:246-256, 1979) except that, in the present study, limited cleavage of the phage RNA was necessary to activate these sites. More extensive fragmentation of R17 RNA permitted wheat germ ribosomes to bind and protect a great many additional sites. Thus, presence of an (exposed) 5'-terminus on an RNA molecule appears to be necessary and sufficient for attachment of eucaryotic ribosomes.
Full text
PDF

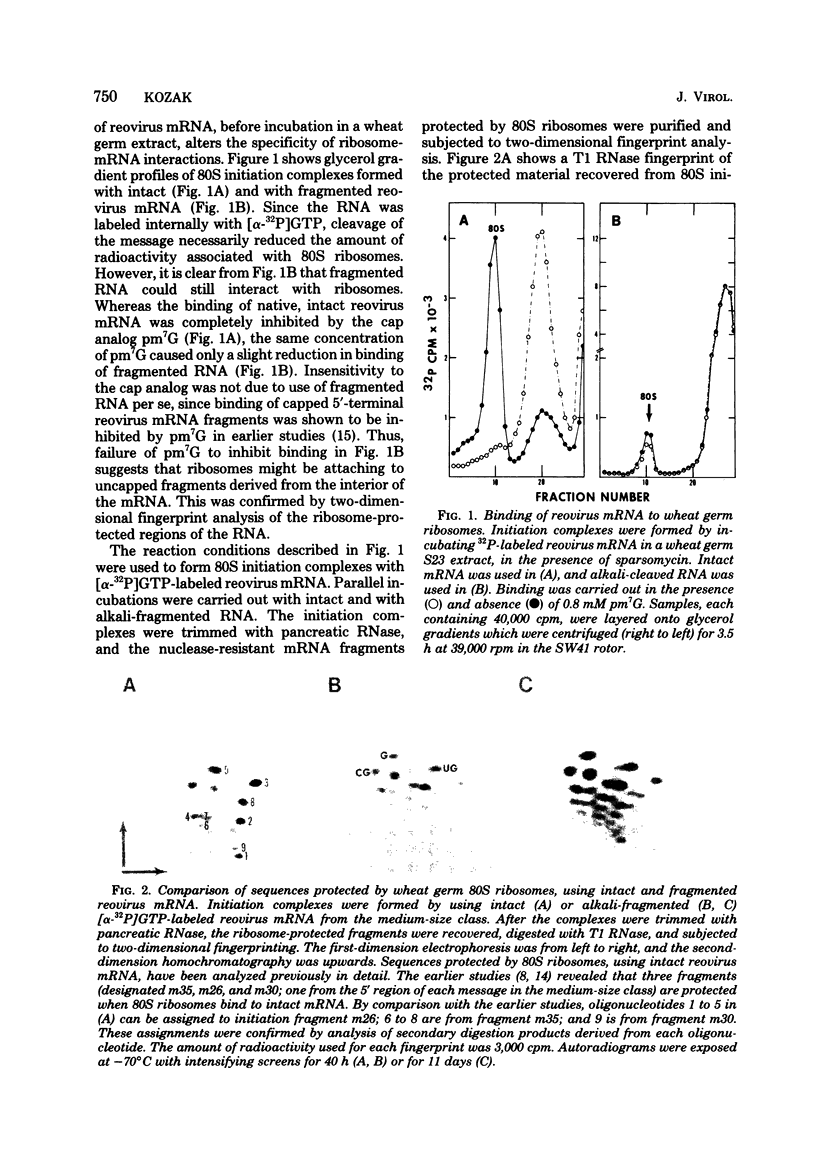



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. F., Steitz J. A., Anderson C. W., Model P. Binding of mammalian ribosomes to MS2 phage RNA reveals an overlapping gene encoding a lysis function. Cell. 1979 Oct;18(2):247–256. doi: 10.1016/0092-8674(79)90044-8. [DOI] [PubMed] [Google Scholar]
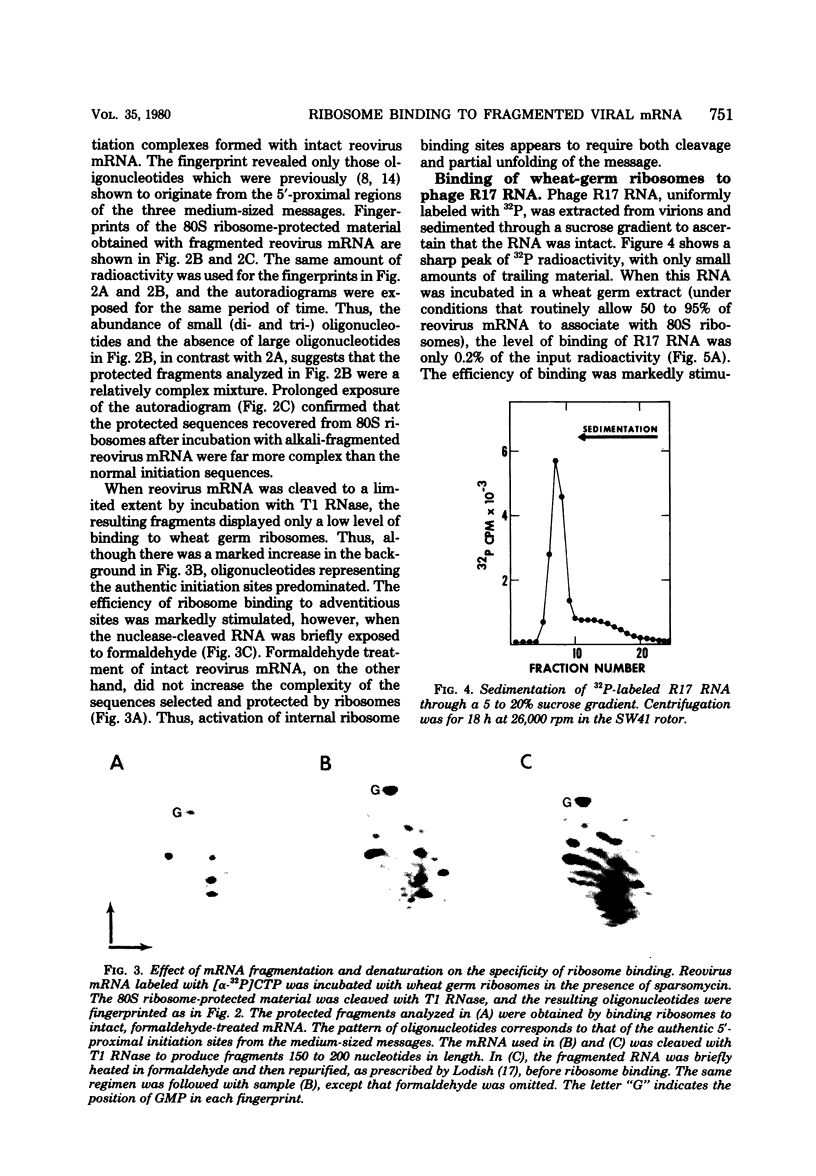
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Lavi S., Shatkin A. J. Synthesis of all the gene products of the reovirus genome in vivo and in vitro. Cell. 1975 Feb;4(2):173–180. doi: 10.1016/0092-8674(75)90124-5. [DOI] [PubMed] [Google Scholar]
- Dasgupta R., Shih D. S., Saris C., Kaesberg P. Nucleotide sequence of a viral RNA fragment that binds to eukaryotic ribosomes. Nature. 1975 Aug 21;256(5519):624–628. doi: 10.1038/256624a0. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Kaesberg P. Translation of virus mRNA: synthesis of bacteriophage Q beta proteins in a cell-free extract from wheat embryo. J Virol. 1973 Dec;12(6):1434–1441. doi: 10.1128/jvi.12.6.1434-1441.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Duerinck F., Haegmean G., Merregaert J., Jou W. M., Raeymakers A., Volckaert G., Ysebaert M., Van de Kerckhove J. A-protein gene of bacteriophage MS2. Nature. 1975 Jul 24;256(5515):273–278. doi: 10.1038/256273a0. [DOI] [PubMed] [Google Scholar]
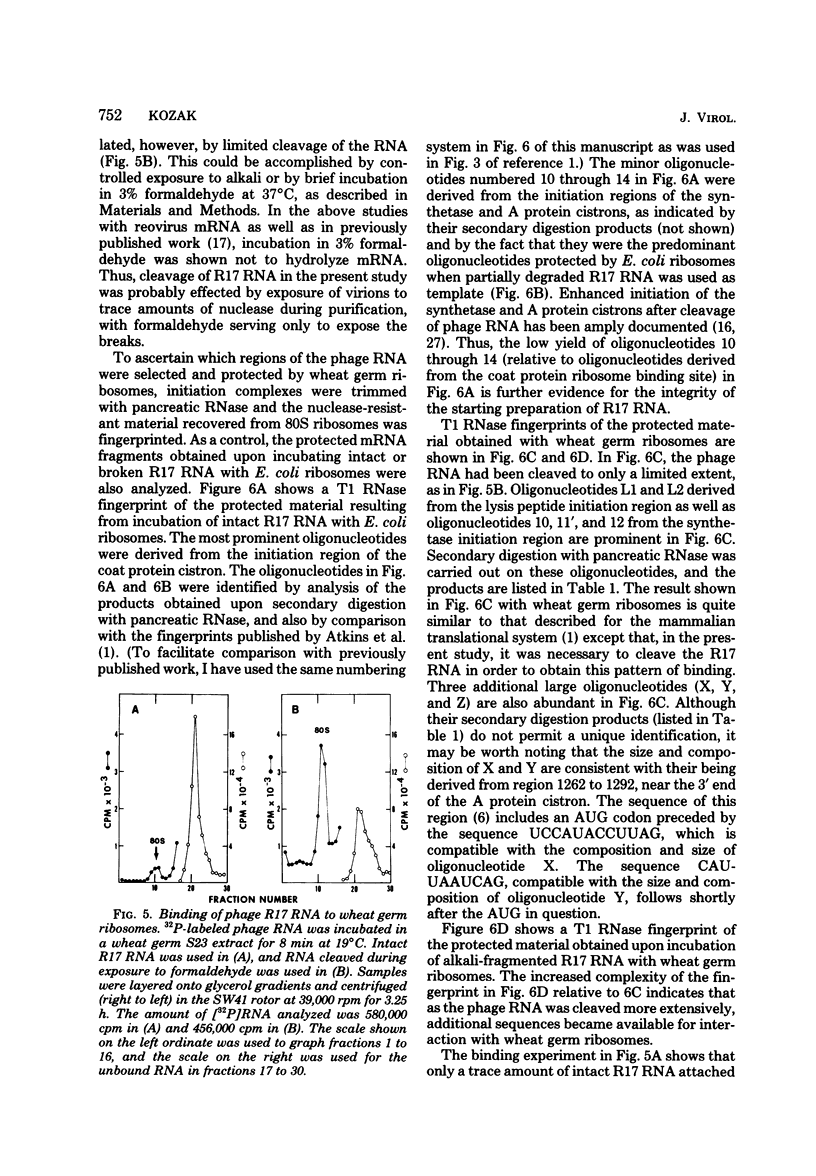
- Jacobson A. B., Spahr P. F. Studies on the secondary structure of single-stranded RNA from the bacteriophage MS2. II Analysis of the RNase IV cleavage products. J Mol Biol. 1977 Sep 25;115(3):279–294. doi: 10.1016/0022-2836(77)90155-3. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. Inability of circular mRNA to attach to eukaryotic ribosomes. Nature. 1979 Jul 5;280(5717):82–85. doi: 10.1038/280082a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Influence of mRNA secondary structure on binding and migration of 40S ribosomal subunits. Cell. 1980 Jan;19(1):79–90. doi: 10.1016/0092-8674(80)90390-6. [DOI] [PubMed] [Google Scholar]
- Kozak M. Migration of 40 S ribosomal subunits on messenger RNA when initiation is perturbed by lowering magnesium or adding drugs. J Biol Chem. 1979 Jun 10;254(11):4731–4738. [PubMed] [Google Scholar]
- Kozak M. Nucleotide sequences of 5'-terminal ribosome-protected initiation regions from two reovirus messages. Nature. 1977 Sep 29;269(5627):391–394. doi: 10.1038/269390a0. [DOI] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Characterization of ribosome-protected fragments from reovirus messenger RNA. J Biol Chem. 1976 Jul 25;251(14):4259–4266. [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Identification of features in 5' terminal fragments from reovirus mRNA which are important for ribosome binding. Cell. 1978 Jan;13(1):201–212. doi: 10.1016/0092-8674(78)90150-2. [DOI] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Sequences of two 5'-terminal ribosome-protected fragments from reovirus messenger RNAs. J Mol Biol. 1977 May 5;112(1):75–96. doi: 10.1016/s0022-2836(77)80157-5. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Independent translation of the genes of bacteriophage f2 RNA. J Mol Biol. 1968 Mar 28;32(3):681–685. doi: 10.1016/0022-2836(68)90351-3. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Secondary structure of bacteriophage f2 ribonucleic acid and the initiation of in vitro protein biosynthesis. J Mol Biol. 1970 Jun 28;50(3):689–702. doi: 10.1016/0022-2836(70)90093-8. [DOI] [PubMed] [Google Scholar]
- Min Jou W., Haegeman G., Ysebaert M., Fiers W. Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein. Nature. 1972 May 12;237(5350):82–88. doi: 10.1038/237082a0. [DOI] [PubMed] [Google Scholar]
- Morrison T. G., Lodish H. F. Translation of bacteriophage Q RNA by cytoplasmic extracts of mammalian cells. Proc Natl Acad Sci U S A. 1973 Feb;70(2):315–319. doi: 10.1073/pnas.70.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucha E., Harvey R., Smith A. E. Cell-free synthesis of simian virus 40 T-antigens. J Virol. 1978 Oct;28(1):154–170. doi: 10.1128/jvi.28.1.154-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Translation of fragmented viral RNA in vitro: initiation at multiple sites. FEBS Lett. 1979 Apr 1;100(1):195–199. doi: 10.1016/0014-5793(79)81162-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Paterson B. M. Efficient cap-dependent translation of polycistronic prokaryotic mRNAs is restricted to the first gene in the operon. Nature. 1979 Jun 21;279(5715):696–701. doi: 10.1038/279696a0. [DOI] [PubMed] [Google Scholar]
- Sangar D. V., Black D. N., Rowlands D. J., Harris T. J., Brown F. Location of the initiation site for protein synthesis on foot-and-mouth disease virus RNA by in vitro translation of defined fragments of the RNA. J Virol. 1980 Jan;33(1):59–68. doi: 10.1128/jvi.33.1.59-68.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T., Gesteland R. F., Spahr P. F. Translation of bacteriophage R17 and Qbeta RNA in a mammalian cell-free system. J Mol Biol. 1973 Apr 15;75(3):575–578. doi: 10.1016/0022-2836(73)90462-2. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Clark J. M., Jr Effect of capping upon the mRNA properties of satellite tobacco necrosis virus ribonucleic acid. Biochemistry. 1979 Apr 3;18(7):1366–1371. doi: 10.1021/bi00574a037. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Discriminatory ribosome rebinding of isolated regions of protein synthesis initiation from the ribonucleic acid of bacteriophage R17. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2605–2609. doi: 10.1073/pnas.70.9.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A. Polypeptide chain initiation: nucleotide sequences of the three ribosomal binding sites in bacteriophage R17 RNA. Nature. 1969 Dec 6;224(5223):957–964. doi: 10.1038/224957a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Prokaryotic ribosome binding sites. Methods Enzymol. 1979;60:311–321. doi: 10.1016/s0076-6879(79)60029-0. [DOI] [PubMed] [Google Scholar]
- Volckaert G., Fiers W. A micromethod for base analysis of 32P-labeled oligoribonulcleotides. Anal Biochem. 1977 Nov;83(1):222–227. doi: 10.1016/0003-2697(77)90530-9. [DOI] [PubMed] [Google Scholar]
- Volckaert G., Fiers W. Micro thin-layer techniques for rapid sequence analysis of 32P-labeled RNA: double digestion and pancreatic ribonuclease analyses. Anal Biochem. 1977 Nov;83(1):228–239. doi: 10.1016/0003-2697(77)90531-0. [DOI] [PubMed] [Google Scholar]