Abstract
Serum amyloid P component (SAP) is a member of pentraxins. Previous studies indicate that SAP exists in human atherosclerotic aortic intima and the plasma SAP levels are associated with cardiovascular disease. In this study, we characterized SAP in normal and atherosclerotic intima, investigated the source of SAP in atherosclerotic lesions, and assessed the effect of SAP on HDL function. Immunohistochemical staining and electroimmunoassay indicated that SAP is not present in normal aortic intima which excludes the possibility that SAP nonspecifically deposits in aortic intima via its binding to microfibrils. Notably, SAP levels are correlated with the severity of atherosclerotic lesions. Fast protein liquid chromatography (FPLC) and western blot analysis revealed that SAP exists in atherosclerotic lesions in multiple forms. Soluble SAP accumulates in the lesions as decamer in free or bound forms via ligand-binding to its ligand(s). Insoluble SAP accumulates in the lesions in covalent-bound forms conjugated to collagen/collagen-like substances via disulfide (-S-S-) bonds. In situ hybridization and RT-PCR analysis revealed that SAP is generated in atherosclerotic lesions, at least partly, by macrophages and smooth muscle cells in neointima. Functional analysis demonstrated that SAP associated with HDL promotes SR-BI-dependent cholesterol efflux and lipid-free SAP enhances ABCA1-dependent cholesterol efflux. In conclusion, our findings demonstrate that SAP is specifically accumulated and expressed in atherosclerotic lesions. SAP may be involved in cholesterol clearance through its role in promoting cholesterol efflux.
Keywords: amyloid P component, pentraxin, HDL, cardiovascular disease, cholesterol efflux, scavenger receptor BI, ABCA1
Introductions
Pentraxins are a group of highly conserved proteins composed of five identical subunits non-covalently associated in a pentameric ring1. They are involved in innate and adaptive immunity and closely related to inflammatory diseases such as atherosclerosis2. Serum amyloid P component (SAP) is a main member of pentraxin family3. It is synthesized by the liver and circulates in human blood at 30-45 mg/L4. SAP is capable of binding a variety of ligands and accumulating evidence indicates that this ligand-binding property may be closely related to its in-vivo functions. For examples, SAP binds to amyloid fibrils, and mice deficient in SAP exhibit delayed development of amyloidosis5, 6; SAP binds to DNA/chromatin, and mice deficient in SAP have impaired clearance of chromatin resulting in autoimmune disease7-9.
SAP shares 70% homology with C-reactive protein (CRP), another main member of pentraxin family. While CRP is a well-established risk factor of cardiovascular disease10, the contribution of SAP to cardiovascular disease is less characterized. Our early studies showed that SAP is present in atherosclerotic lesions, and SAP binds to high density lipoprotein (HDL) and very low density lipoprotein (VLDL) with high affinity, suggesting that SAP is involved in atherosclerosis and lipoprotein metabolism11, 12. Recent studies further revealed that SAP is colocalized with apolipoprotein in human atheroma, and notably, plasma SAP levels are positively associated with cardiovascular disease in the elderly13, 14. While these findings suggest a role of SAP in atherosclerosis, a number of questions, such as whether SAP specifically accumulates in atherosclerotic lesions or universally presents in aortic intima, how SAP accumulates in atherosclerotic lesions, what is the source of SAP in atherosclerotic lesion and what is the possible role of SAP in lipoprotein metabolism, still remain to be elucidated. In this study, we employed immunohistochemical and biochemical assays to answer these questions. We found that SAP is not present in normal human aortic intima, which excludes the possibility that SAP non-specifically deposits in aortic intima via binding to microfibrils. Notably, SAP levels are positively correlated with the severity of atherosclerosis, suggesting that SAP is specifically accumulated in atherosclerotic lesions. Importantly, our study first revealed that SAP can be generated extra-hepatically by macrophages and functional assay indicated that SAP promotes cholesterol efflux, suggesting that SAP may play a role in cholesterol removal from cells.
Materials and Methods
Materials
Rabbit anti-human SAP antiserum was purchased from UCB-Bioproducts; Vecta-stain ABC kit against rabbit IgG from Vector Laboratories; DAKO LSAB kit against goat IgG from Dako A/S; FPLC, Superose 6 HR 10/30 column and HMW gel filtration calibration kit from GE Healthcare; bacterial collagenase from Wako Pure Chemical Industries, purified human SAP, DTT and LPS from Sigma Chemical Co; PCRII vector from Invitrogen.
Adsorption of anti-SAP serum with SAP-deficient human plasma
Early study indicated that rabbit anti-SAP serum reacts with IgG, and generates false positive bands in Western blotting.11 To eliminate these nonspecific reactions, the rabbit anti-SAP serum was mixed with equal volume of SAP-deficient human plasma. The mixture was incubated at 4°C overnight and centrifuged at 12,000g for 10 min. The supernatant was adsorbed with half volume of SAP-deficient human plasma again as described above. The adsorbed anti-SAP serum did not show false positive bands in Western blot and was used for immunohistochemical staining, immunoelectrophoresis and Western blotting.
Collection of aortic specimens
for biochemical analysis, human aortic specimens were obtained from autopsies or operation at the National Cardiovascular Center, Osaka, Japan. The tunica intima was separated from the underlying tunica media along the natural cleavage plane of the internal elastic lamina. The intima was blotted to a filter paper to remove excess moisture, weighed, and stored at −80°C. The tissues were analyzed within one month. The severity of atherosclerosis of the tissues was evaluated. Tissue without thickened intima was considered as normal (n=6), with light yellow lipid deposition was considered as moderate lesions (n=5), with atheromatous plaque, fibrous plaque, calcified plaque and complicated lesion was considered as advanced lesions (n=6). Fifteen paraffin embedded human aortas were used for immunohistochemical study, which were obtained from the Department of Pathology of National Cardiovascular Center. The severity of atherosclerosis of the tissues was evaluated by Mayer’s hematoxylin staining according to the classification of Stary et al15. The tissue without thickened intima was defined as normal (n=6), with type I to III lesions was defined as moderate lesions (n=3), and with type IV to VI lesions was defined as advanced lesions (n=6). The tissue materials used in the present study were obtained in accordance with local rules for collection of samples from human.
Extraction of SAP from aortic intima
Quantitative sequential extracts with CaCl2-Tris-buffered saline (TBS), EDTA-TBS and collagenase digestion were performed as previously described11. Briefly, the intima was cut into small pieces and homogenized for 3 min in a homogenizer (IKA-Ultra-Turrax T25, Janke & Kankel GmbH & Co. KG) with 5 ml/g wet tissue of CaCl2-TBS. The homogenate was incubated for 30 min, and the extract was collected by centrifugation at 20,000g for 15 min. The pellets were washed three times by re-homogenization with the CaCl2-TBS. The supernatant was discarded and the pellets were then extracted with 10 mmol/L EDTA-TBS as described above. After three times washes with 5 mmol/L CaCl2-50 mmol/L Tris-HCl, pH 7.5, the pellets were digested with 5 ml/g wet tissue of 200 U/ml bacterial collagenase at 37°C for 1 h, and the supernatant was collected by centrifugation at 20,000g for 15 min.
Quantification of SAP in aortic intima
The content of intimal SAP was measured by electroimmunoassay as previously described.11 Serial dilutions of normal human plasma were used as standards that were calibrated against purified SAP.
Immunohistochemical detection of SAP in aortic intima
Immunohistochemical staining of SAP in human aortic intima was performed with the streptavidin-biotin method using the DAKO LSAB kit as we previously described.11 The adsorbed rabbit anti-SAP serum was used as the primary antibody. The antiserum adsorbed with purified SAP was used as a negative control. DAB was used as the chromogen.
FPLC analysis of SAP from atherosclerotic aortic intima
The sequential intimal extracts from advanced atherosclerotic aortas were filtered through a 0.45 μm filter, and 200 μl of the samples were applied to a Superose 6 HR 10/30 column, which was equilibrated with 2 mmol/L EDTA-50 mmol/L Tris-saline, pH 7.5. The chromatography was run at 0.5 ml/min, and 0.5 ml of the fractions was collected. The effluent was subjected to electroimmunoassay and Western blot to check the presence of SAP. The column was calibrated using the HMW gel filtration calibration kit.
Cell culture
human mononuclear cells were isolated from heparinized human blood using LymphoPrep. The cells were culture for 2h for monocytes attachment and cultured in RPMI1640 medium containing 10% FBS for 4 days. The cell were treated with/without lipopolysaccharide (LPS, 1ng/ml) and phorbol-12 myristate 13-acetate (PMA, 50ng/ml) for 12h and then harvested for RT-PCR analysis. Human umbilical artery smooth muscle cells (HUA-SMC) were cultured in DMED medium containing 20% FCS and 10ng/ml of β–FGF. The cells were treated with/without LPS (10ng/ml) and PMA (50ng/ml) for 12h and then harvested for RT-PCR analysis.
RT-PCR
Total RNA was isolated with TRIzol reagent (Invitrogen) and treated with DNase to remove genomic DNA. RT-PCR was performed with a one-step RT-PCR kit from Pharmacia (GE) using forward primer 5′-CCTCATCCTGGTCACTGCTT and reverse primer 5′-GAGAAGAGGCTGTAGGCACG.
In situ hybridization
A 401bp human SAP cDNA was subcloned into pCRII vector (Invitrogen) by PCR method using human SAP cDNA (ATCC 59560) as the template with forward primer 5′-TGATCACACCGCTGGAGAAG and reverse primer 5′-CCCAATCTCTCCCACAAAGG. The direction of insert was checked by sequencing. The antisense and sense probes were generated with T7 primer and SP6 primer, respectively, and labeled with UTP-digoxigenin (Boehringer Mannheim Biochemica, Mannheim, Germany) according to the manufacturer’s instructions. Hybridization of SAP mRNA was performed at 50°C for 16 h, and the signal was detected using a nucleic acid detection kit (Boehringer) as previously described16. Hybridization with sense probe was used as negative control.
Cholesterol efflux assay
Cellular cholesterol efflux was determined essentially as described17. Briefly, cells (about 70% confluent) in 12-well plates were labeled with 0.2 μCi/ml [3H]cholesterol (35–50 Ci/mmol, Amersham Biosciences) in medium for 48 h. For ABCA1-dependent efflux, J774 cells were incubated in the presence or absence of 0.3 mM cAMP for 18 h. Addition of cAMP to J774 cells stimulates ABCA1 expression so that it is used to determine the ABCA1-dependent cholesterol efflux18,19. Cells were then washed five times with PBS containing 0.1% BSA and equilibrated in serum-free medium containing 0.2% fatty acid-free BSA for 5h. Thereafter, cells were incubated for 16 h at 37 °C in media with or without ligands, as indicated in the legends to figures. For SR-BI dependent efflux, cells were washed five times with the wash buffer and equilibrated for 16 h as mentioned above. Cells were then incubated with ligands for 5 h at 37 °C. Following incubation, medium was collected, and cells were washed three times with PBS containing 0.1% fatty acid-free BSA and then three times with PBS only at 4 °C. Radioactivity in the media was measured directly in a β liquid scintillation counter (PerkinElmer, CT). Cellular lipid was extracted with hexane/isopropyl alcohol (3:2 v/v) for 30 min at room temperature and counted for radioactivity. Lipid-extracted cells were solubilized in 0.1M NaOH for protein determination. Efflux of cellular [3H]cholesterol to media is expressed as a percentage of total radioactivity in medium and cells. Efflux was calculated as the percentage of counts in the medium to the counts in the medium and cells together.
Statistical Analysis
Values are reported as the mean ± standard deviation (SD). Student’s t-test was employed for statistical analysis. A value of P<0.05 was considered significant.
Results
SAP presents in atherosclerotic aortic lesions but not in normal aorta
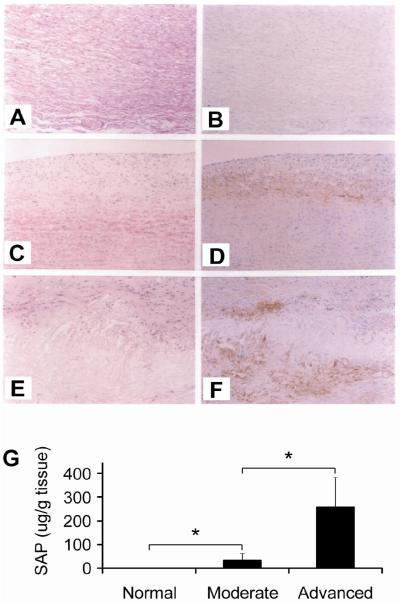
We performed immunohistochemical staining on aorta with different stages of atherosclerosis. No positive staining was found in normal aortic intima (Figure 1A and B). Positive stainings were observed in the deeper site of the intima with moderate atherosclerotic lesions (Figure 1C and D) and more positive stainings were found in the advanced atherosclerotic aortic intima (Figure 1E and F). SAP was localized at the atheromatous core and the surrounding tissue of the core. When using SAP-adsorbed antiserum as negative control, no positive staining was found (data not shown).
Figure. 1. Serum amyloid P component (SAP) in normal and atherosclerotic aortic intima.
A to F. Immunohistochemical staining of SAP. Aortas from normal, moderate and advanced atherosclerotic patients were stained with hematoxylin/eosin (A, C and E) or immunostained with anti-SAP serum (B, D and F). Positive immunostainings are identified as a brown reaction product. Original magnification, x50. G. Quantification of SAP in normal and atherosclerotic intima. SAP content in normal, moderate and advanced atherosclerotic intima was quantified with electroimmunoassay. *p < 0.05, moderate versus normal and advanced versus moderate.
We then quantified SAP in normal and atherosclerotic aortic intima using electroimmunoassay. As shown in Figure 1G, no SAP was detected in normal aortic intima. SAP was found in intima of moderate atherosclerotic lesions with a value of 30.5±29.3 μg/g wet tissue (p = 0.018 versus normal intima) and markedly increased in advanced lesions with a value of 258.1±143.6 μg/g wet tissue (p = 0.007 versus normal intima and p = 0.010 versus moderate lesions).
Characterization of SAP in atherosclerotic aortic intima
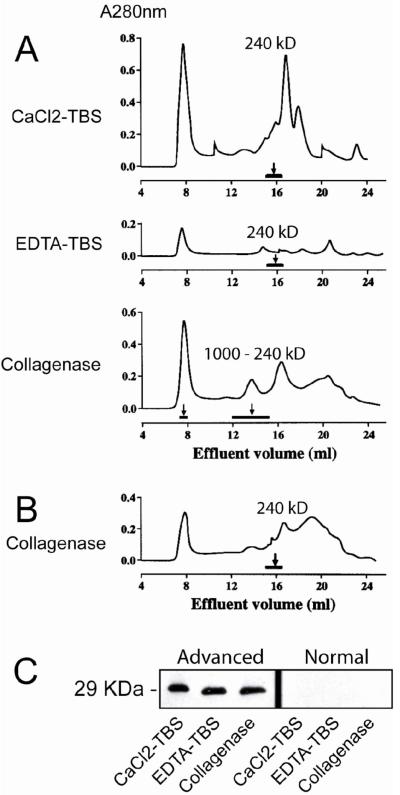
Our early study indicated that SAP exists in atherosclerotic lesions in multiple forms11. To further characterize SAP in the lesions, we sequentially extracted SAP with three different buffers: CaCl2-TBS buffer which extracts free SAP; EDTA-TBS buffer which extracts SAP bound to intima in a calcium-dependent manner and collagenase buffer which extracts SAP that covalently associates with collagen. We found that about 40% appeared in the CaCl2-TBS fraction, 30% in the EDTA-TBS fraction, and 30% in the collagenase digestion fraction. We then applied the sequentially extracted fractions to FPLC equipped with a Superose 6 HR 10/30 column. As shown in Figure 2A, SAP from CaCl2-TBS and EDTA-TBS fractions eluted as a single peak at 240 kD, indicating that SAP in these fractions exists in decamer form. However, SAP from collagenase digestion fraction eluted in two fractions. A small portion of SAP appeared in void volume (MW>2,000 kD), and the majority of SAP appeared as a broad peak from 240 kD to 1000 kD. When the collagenase digested fraction was reduced with dithiothreitol (DTT, 2mM), SAP was eluted at 240 kD (Figure 2B). When the effluents were applied to Western blot in reduced condition, SAP appeared at 29 kD position as SAP monomer did (Figure 2C). These results indicate that SAP in collagenase digested fraction exists in bound form, covalently conjugates to collagen/collagen-like substances via disulfide (-S-S-) bonds. We also applied samples extracted from normal aorta to Western blot. The same as electroimmunoassay, no sap was detected (Figure 2C).
Figure 2. Characterization of SAP in atherosclerotic aortic intima.
A and B. Gel filtration analysis of SAP in atherosclerotic lesions. The sequential intimal extracts from an advanced atherosclerotic aorta by CaCl2-TBS, EDTA-TBS and collagenase digestion were applied to FPLC equipped with a Superose 6 HR 10/30 column. The fractions were monitored by UV A280nm, and SAP in each fraction was detected by electroimmunoassay and the presence of SAP was indicated by horizontal bar (A). The collagenase digested extract was also reduced with 2mM DTT and subjected to FPLC analysis (B). C. SDS-PAGE-Western blot analysis of SAP in atherosclerotic or normal aorta. The sequential intimal extracts from an advanced atherosclerotic aorta or a normal aorta were applied to reduced SDS-PAGE and the SAP in sequential extracts was analyzed by Western blot with anti-SAP antiserum.
Expression of SAP in atherosclerotic lesions
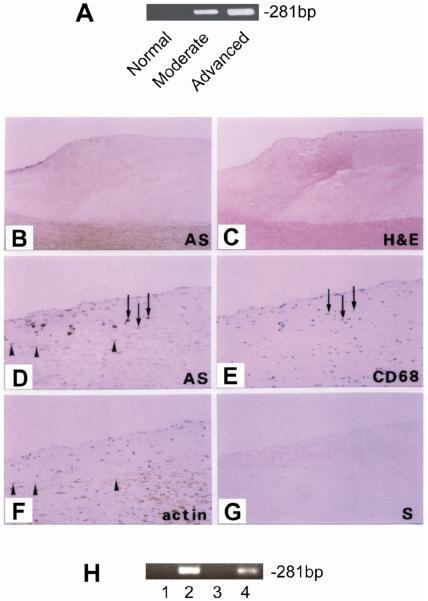
We isolated RNA from normal and atherosclerotic aortic intima and performed RT-PCR to determine whether SAP can be generated in atherosclerotic aortic intima. As shown in Figure 3A, SAP mRNA was clearly detected in atherosclerotic intima but not in normal intima.
Figure 3. Expression of SAP in atherosclerotic lesions.
A. RT-PCR of SAP using RNA isolated from normal and atherosclerotic intima. B to G. In Situ hybridization. Sections of aorta with advanced lesions were subjected to in situ hybridization using UTP-labeled human SAP antisense probe (panel B, x20 and panel D, x100) or sense probe (panel G, x100). Constitutive sections were also immunostained with anti-CD68 (panel E, x100) or anti-alpha smooth muscle actin (panel F, x100). Arrows indicate macrophages and arrowheads indicate smooth muscle cells. H. Expression of SAP in human monocytes and smooth muscle cells. Human monocytes were treated with (lane 1) or without (lane 2) LPS (1ng/ml) and PMA (50ng/ml) for 12h and then harvested for RT-PCR analysis. Human smooth muscle cells were treated with (lane 3) or without (lane 4) LPS (10ng/ml) and PMA (50ng/ml) for 12h and then harvested for RT-PCR analysis.
To further determine which type of cells produce SAP in atherosclerotic aortic intima, section of advanced atherosclerotic lesions was subjected to in situ hybridization using UTP-digoxigenin-labeled human SAP riboprobes. As illustrated in Figure 3B and 3D, SAP mRNA expressing cells were identified in the neointima near atheromatous plaque. No positive staining was found when sense probes were used (Figure 3G). To further identify the nature of the SAP expressing cells, consecutive sections were immunostained with anti-CD68 antibody or anti-alpha smooth muscle actin antibody. SAP expressing cells showed positive staining for CD68 (Figure 3E) and actin (Figure 3F), indicating that at least part of SAP is produced by macrophages and smooth muscle cells in the neointima. We also assessed SAP expression in human macrophages and aortic smooth muscle cells using RT-PCR. As shown in Figure 3H, SAP mRNA expression was detected in both macrophages and aortic smooth muscle cells that were treated with LPS/PMA.
SAP promotes cholesterol efflux
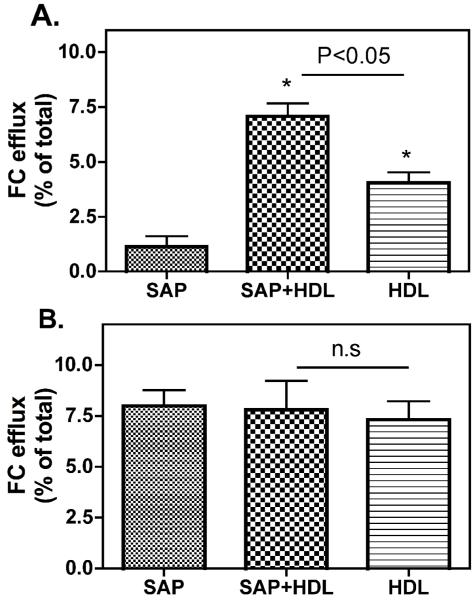
We previously demonstrated that SAP binds to HDL with high affinity.12 Considering that the function of SAP may be related to its ligand binding, we tested the effect of SAP-HDL association on HDL-mediated cholesterol efflux. We employed CHO-SR-BI and CHO-A7 cells, and J774 cells stimulated with/without cAMP to elucidate the effect of SAP on SR-BI-dependent and ABCA1-dependent cholesterol efflux, respectively. cAMP stimulates the expression of ABCA1 in J774 cells so that we used this cell system to determine ABCA1-depennet cholesterol efflux18, 19. As indicated in Figure 4A, lipid-free SAP (even at 40 μg/ml) did not show SR-BI-dependent cellular cholesterol efflux in CHO cells expressing SR-BI. SR-BI-dependent cholesterol efflux was observed in the presence of HDL, and importantly, compared to HDL alone, HDL incubated with SAP (SAP: 20 μg/ml + HDL: 40 μg/ml) markedly enhanced the SR-BI-dependent cholesterol efflux, indicating SAP can promote HDL-mediated cholesterol efflux. Neither lipid-free SAP nor SAP associated with HDL enhanced cholesterol efflux in CHO-A7 cells (data not shown), indicating that SAP promotes HDL-mediated cholesterol efflux in a SR-BI-dependent manner. Interestingly, as indicated in Figure 4B, lipid-free SAP promoted cholesterol efflux from J774 cells stimulated with cAMP, but not from J774 cells without cAMP treatment, and the extend of cholesterol efflux was similar to that of HDL did, which indicates that lipid-free SAP is capable of promoting ABCA1-dependnet cholesterol efflux. However, SAP associated with HDL did not further enhance ABCA1-dependent cholesterol efflux compared to HDL alone, indicating that HDL associated with SAP is not a better cellular cholesterol acceptor for ABCA1-dependent cholesterol efflux than HDL.
Figure 4. Effect of SAP on cholesterol efflux.
A. SR-BI-dependent cholesterol efflux to SAP. CHO-SR-BI and control CHO-A7 cells were labeled with 0.2 μCi/ml [3H]cholesterol for 48 h, equilibrated in cholesterol free medium for 16 h, and then incubated with SAP, SAP + HDL3 or HDL3 at 37 °C for 5 h to determine cellular efflux as described in “Material and Methods”. Efflux of free cholesterol into the media is expressed as percentage of the total radioactivity in the media and cells. The efflux values were subtracted from blank values obtained by incubating cells with albumin containing efflux medium. SR-BI-specific values were calculated as the difference between the efflux values in CHO-SRBI cells and control CHO-A7 cells. Values shown are mean ± SD of triplicate determinations. B. ABCA1-dependent cholesterol efflux in macrophages. J774 cells were labeled with 0.2 μCi/ml [3H]cholesterol in medium for 48 h. Cellular ABCA1 expression was stimulated by incubation of J774 cells with 0.3mM of cAMP for 16 h. Efflux experiments were performed at 37 °C by incubating cells with different ligands for 16 h. The efflux values were subtracted from blank values obtained by incubating cells with albumin containing efflux medium. ABCA1-specific efflux was calculated by the difference between J774-cAMP and J774 cells. Values shown are mean ± SD of triplicate determinations.
Discussion
In this study, we extend our previous observation and demonstrate that SAP is specially accumulated and expressed in human atherosclerotic intima. We further reveal that SAP associated with HDL promotes SR-BI-dependent cholesterol efflux and lipid-free SAP enhances ABCA1-dependent cholesterol efflux.
Using immunohistochemical and electroimmunological assays we showed that SAP is not present in normal aortic intima, which excludes the possibility that SAP nonspecifically deposits in intima through its binding to elastic membrane. Importantly, we showed that the content of intimal SAP is positively correlated with the severity of atherosclerosis. We further revealed that SAP exists in the atherosclerotic aortic intima in three forms. SAP extracted by CaCl2-TBS buffer exists in the intima in free form as decamer; SAP extracted by EDTA-TBS buffer exists in the intima as ligand-binding form via calcium-dependent intermolecular interaction with its ligand(s). SAP has been shown to bind to a variety of ligands including IgG, complement C1 and lipoproteins. These molecules may function as ligands of SAP that recruits SAP to atherosclerotic lesions. SAP extracted by collagenase digestion exists as complex forms that covalently conjugated to collagen/collagen-like substances. Upon Western blot in reduced condition, the collagenase digestible SAP appeared at SAP monomer position, suggesting that SAP binds to the collagen/collagen-like substances via disulfide (S-S) bonds.
Using in situ analysis and immunohistochemical assay we demonstrated that SAP is produced in the atherosclerotic aortic intima by macrophages and smooth muscle cells. This finding indicates that, in addition to coming from circulating SAP that produced by the liver, SAP in the atherosclerotic lesions can potentially be generated locally. To the best of our knowledge, this was the first evidence showing that SAP is produced by non-hepatic cells.
Using cholesterol efflux assay, we demonstrated that lipid-free SAP and SAP associated HDL promote cellular cholesterol efflux in a SR-BI and ABCA1 dependent manner. These data suggest that SAP may prevent lipid accumulation in macrophages. Under pathological conditions such as atherosclerosis which is frequently associated with decreased HDL levels, SAP may compensate for the function of apoA-I/HDL in cellular cholesterol efflux and may even serve as a crucial factor in limiting lipid accumulation and foam cell formation. Therefore, our future studies will be focused on the in depth mechanistic studies regarding to the function of SAP in atherosclerotic lesion formation by performing the gain of function or loss of function studies.
In summary, our findings demonstrate that SAP is specifically accumulated in atherosclerotic lesions. The source of SAP in atherosclerotic lesions includes circulating SAP that binds to its ligands accumulated in atherosclerotic lesions and/or local SAP generated by macrophages and smooth muscle cells in neointima. SAP may participate in cholesterol removal from macrophages through its role in promoting cholesterol efflux.
Acknowledgements
We thank the members of the Kentucky Pediatric Research Institute for invaluable advice and assistance. This work was supported by grants from American Heart Association (0530241N), NIH (R01GM085231 and 3R01GM085231-02S1) and Children’s Miracle Network.
Abbreviations
- DTT
dithiothreitol
- FPLC
fast protein liquid chromatography
- LPS
lipopolysaccharide
- PMA
phorbol-12 myristate 13-acetate
- SR-BI
scavenger receptor BI
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gewurz H, Zhang X-H, Lint TF. Structure and function of the pentraxins. Current Opinion in Immunology. 1995;7:54–64. doi: 10.1016/0952-7915(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 2.Manfredi AA, Rovere-Querini P, Bottazzi B, Garlanda C, Mantovani A. Pentraxins, humoral innate immunity and tissue injury. Current Opinion in Immunology. 2008;20:538–544. doi: 10.1016/j.coi.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Emsley J, White HE, O’Hara BP, Oliva G, Srinivasan N, Tickle IJ, Blundell TL, Pepys MB, Wood SP. Structure of pentameric human serum amyloid P component. Nature. 1994;367:338–345. doi: 10.1038/367338a0. [DOI] [PubMed] [Google Scholar]
- 4.Pepys MB, Baltz ML, de Beer FC, Dyck RF, Holford S, Breathnach SM, Black MM, Tribe CR, Evans DJ, Feinstein A. Biology of serum amyloid P component. Ann N Y Acad Sci. 1982;389:286–298. doi: 10.1111/j.1749-6632.1982.tb22144.x. [DOI] [PubMed] [Google Scholar]
- 5.Pepys MB, Dyck RF, de Beer FC, Skinner M, Cohen AS. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol. 1979;38:284–293. [PMC free article] [PubMed] [Google Scholar]
- 6.Botto M, Hawkins PN, Bickerstaff MC, Herbert J, Bygrave AE, McBride A, Hutchinson WL, Tennent GA, Walport MJ, Pepys MB. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene. Nat Med. 1997;3:855–859. doi: 10.1038/nm0897-855. [DOI] [PubMed] [Google Scholar]
- 7.Breathnach SM, Kofler H, Sepp N, Ashworth J, Woodrow D, Pepys MB, Hintner H. Serum amyloid P component binds to cell nuclei in vitro and to in vivo deposits of extracellular chromatin in systemic lupus erythematosus. J Exp Med. 1989;170:1433–1438. doi: 10.1084/jem.170.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pepys MB, Butler PJ. Serum amyloid P component is the major calcium-dependent specific DNA binding protein of the serum. Biochem Biophys Res Commun. 1987;148:308–313. doi: 10.1016/0006-291x(87)91111-9. [DOI] [PubMed] [Google Scholar]
- 9.Bickerstaff MC, Botto M, Hutchinson WL, Herbert J, Tennent GA, Bybee A, Mitchell DA, Cook HT, Butler PJ, Walport MJ, Pepys MB. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat Med. 1999;5:694–697. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- 10.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-Reactive Protein as a Risk Factor for Coronary Heart Disease: A Systematic Review and Meta-analyses for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2009;151:483–495. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 11.Li XA, Hatanaka K, Ishibashi-Ueda H, Yutani C, Yamamoto A. Characterization of serum amyloid P component from human aortic atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1995;15:252–257. doi: 10.1161/01.atv.15.2.252. [DOI] [PubMed] [Google Scholar]
- 12.Li XA, Yutani C, Shimokado K. Serum amyloid P component associates with high density lipoprotein as well as very low density lipoprotein but not with low density lipoprotein. Biochem Biophys Res Commun. 1998;244:249–252. doi: 10.1006/bbrc.1998.8248. [DOI] [PubMed] [Google Scholar]
- 13.Jenny NS, Arnold AM, Kuller LH, Tracy RP, Psaty BM. Serum Amyloid P and Cardiovascular Disease in Older Men and Women: Results from the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2007;27:352–358. doi: 10.1161/01.ATV.0000254150.97741.fe. [DOI] [PubMed] [Google Scholar]
- 14.Stewart CR, Haw A, III, Lopez R, McDonald TO, Callaghan JM, McConville MJ, Moore KJ, Howlett GJ, O’Brien KD. Serum amyloid P colocalizes with apolipoproteins in human atheroma: functional implications. J. Lipid Res. 2007;48:2162–2171. doi: 10.1194/jlr.M700098-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr., Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 16.Sato M, Sugano N, Ohzono K, Nomura S, Kitamura Y, Tsukamoto Y, Ogawa S. Apoptosis and expression of stress protein (ORP150, HO1) during development of ischaemic osteonecrosis in the rat. J Bone Joint Surg Br. 2001;83-B:751–759. doi: 10.1302/0301-620x.83b5.10801. [DOI] [PubMed] [Google Scholar]
- 17.van der Westhuyzen DR, Cai L, de Beer MC, de Beer FC. Serum amyloid A promotes cholesterol efflux mediated by scavenger receptor B-I. J Biol Chem. 2005;280:35890–35895. doi: 10.1074/jbc.M505685200. [DOI] [PubMed] [Google Scholar]
- 18.Haidar B, Denis M, Krimbou L, Marcil M, Genest J., Jr. cAMP induces ABCA1 phosphorylation activity and promotes cholesterol efflux from fibroblasts. J Lipid Res. 2002;43:2087–2094. doi: 10.1194/jlr.m200235-jlr200. [DOI] [PubMed] [Google Scholar]
- 19.Fournier N, Francone O, Rothblat G, Goudouneche D, Cambillau M, Kellner-Weibel G, Robinet P, Royer L, Moatti N, Simon A, Paul JL. Enhanced efflux of cholesterol from ABCA1-expressing macrophages to serum from type IV hypertriglyceridemic subjects. Atherosclerosis. 2003;171:287–293. doi: 10.1016/j.atherosclerosis.2003.08.011. [DOI] [PubMed] [Google Scholar]