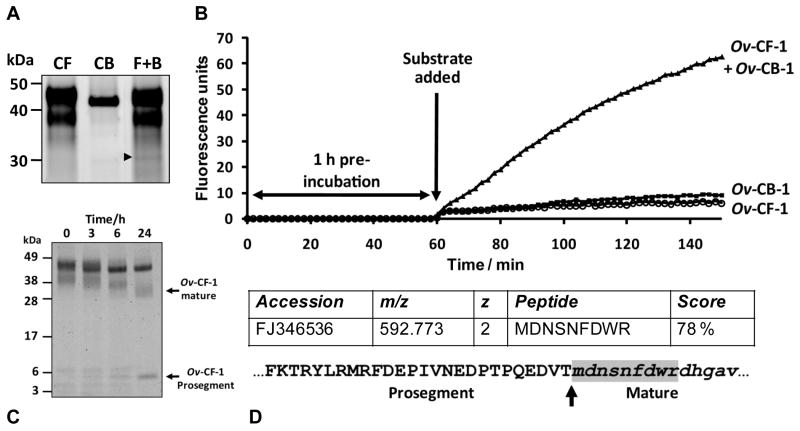
Figure 5.
Exogenous activation of Ov-CF-1 by Ov-CB-1. Purified recombinant Ov-CF-1 was pre-incubated with Ov-CB-1 in 0.1 M sodium acetate containing 1 mM EDTA and 1 mM DTT (pH 5.5) for 1 h at 37°C. Each recombinant was also incubated alone as control reactions. (A) Reactions were analysed on 4-12 % Bis-Tris gels. A band with a molecular mass of ∼ 30 kDa (arrowhead) consistent with the mass of mature cathepsin F, that appeared when Ov-CF-1 and Ov-CB-1 were co-incubated was confirmed as a processed variant of Ov-CF-1 by nanoLC-ESI-MS/MS. (B) Following the 1 h pre-incubation step, the dipeptide substrate Z-Leu-Arg-NHMec was added and the reaction was assayed for proteolytic activity by monitoring the release of the fluorogenic leaving group (-NHMec) over 90 min at 37°C. The specific activity of Ov-CF-1 against Z-Leu-Arg-NHMec increased over the course of the assay when the enzyme was pre-incubated with Ov-CB-1. (C) Prolonged incubation (up to 24 h) of Ov-CF-1 with Ov-CB-1 resulted in a SDS-PAGE profile consistent with the molecular sizes of the Ov-CF-1 mature enzyme and liberated prosegment that was identical to that observed when Ov-CF-1 was trans-activated by exogenous FhCL1 (Pinlaor et al., 2009). (D) The ∼ 30 kDa processed variant of Ov-CF-1 (A, arrowhead) was digested with trypsin and analysed by nano-LC-ESI-MS/MS. A high-scoring putative N-terminal peptide MDNSNFDWR (m/z 592.773) was detected (shaded in grey) and indicates that Ov-CB-1 removes the prosegment of Ov-CF-1 via cleavage at Val-1-Asp-1 ↓ Met1 (arrow) which is also the site used during Ov-CF-1 autocatalysis and exogenous cleavage of the Ov-CF-1 zymogen by FhCL1 (Pinlaor et al., 2009). An annotated MS/MS spectra for the 592.773 precursor ion is shown in Supplementary Figure 1.