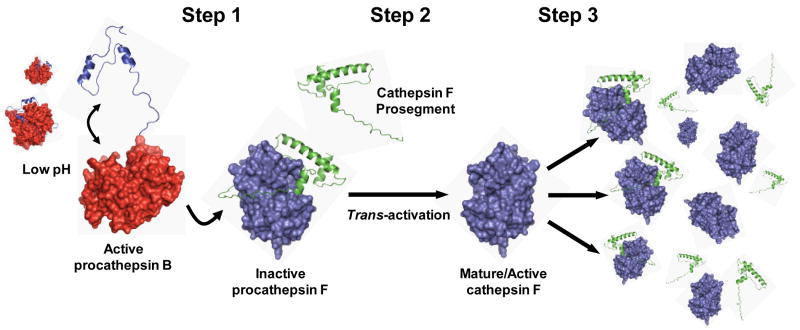
Figure 9.
Proposal for a three-step mechanism of cathepsin F trans-activation in O. viverrini. Step 1: under the acidic microenvironment of the O. viverrini gut, the short pH-sensitive prosegment of cathepsin B undergoes conformational relaxation and dissociates from the mature domain sufficient to allow the zymogen to become active. Step 2: subsequently, in a bi-molecular process, a small number of cathepsin F zymogens are trans-activated by the active cathepsin B zymogens which remove the cathepsin F prosegment via cleavage at Val-1-Asp-1↓Met1 at the junction of the prosegment and mature domain. Step 3: the rapid cleavage of prosegments from other cathepsin F zymogens by the trans-activated cathepsin F molecules again through cleavage at Val-1-Asp-1↓Met1. Molecular models of Ov-CB-1 and Ov-CF-1 were established (for illustrative purposes only) by the SWISS-MODEL homology modelling pipeline (http://swissmodel.expasy.org) using the atomic structures of human procathepsin B (PBD ID: 3PBH) and F. hepatica cathepsin L1 (PBD ID: 2O6X) as templates, respectively. Figures were produced with Pymol (http://www.pymol.org).