Abstract
Fullerenes are carbon cages of variable size that can be derivatized with various side chain moieties resulting in compounds that are being developed into nanomedicines. While fullerene use in several pre-clinical in vitro and in vivo models of disease has demonstrated their potential as diagnostic and therapeutic agents, little is known about how they enter cells, what organelles they target, and the time course for their cellular deposition. Fullerenes (C70) that have previously been shown to be potent inhibitors of mast cell (MC)-mediated allergic inflammation were conjugated with Texas Red (TR) and used in conjunction with confocal microscopy to determine mechanisms of uptake, the organelle localization, and the duration they can be detected in situ. We show C70-TR are non-specifically endocytosed into MC where they are shuttled throughout the cytoplasm, lysosomes, mitochondria, and into endoplasmic reticulum at different times. No nuclear or secretory granule localization was observed. The C70-TR remained detectable within cells at one week. These studies show MC endocytose fullerenes where they are shuttled to organelles involved with calcium and reactive oxygen species (ROS) production which may explain their efficacy as cellular inhibitors.
Keywords: Fullerene, Nanomedicine, Mast cell, Intracellular trafficking
Introduction
The use of nanomaterials to diagnose and treat disease has been a goal for scientists ever since the advent of methods to manipulate molecules at the nanometer level. Fullerenes are one type of nanomaterial that are being investigated for diagnostic and therapeutic modalities (1). Our studies have previously suggested that certain derivatives possess anti-inflammation properties (2;3) and other groups have demonstrated their use in neurological disorders (4). We additionally showed certain fullerenes could potentiate hair growth (5). However, their use as new nanomedicines has not yet fully been realized due to the lack of clearly identified candidates for a particular disease. Once these candidates are more thoroughly characterized, relevant pharmacological and biodistribution data must be assessed. Fullerenes full capabilities as new nanomedicines has been additionally hampered by the inconsistency in how the water insoluble fullerenes are derivatized into biologically-acceptable agents; most studies to date have used highly impure and uncharacterized material which makes interpretation of data difficult.
To begin to address the issue of in situ biodistribution, previously characterized fullerene derivatives that have been shown to have anti-inflammatory activity (3) were modified by chemically adding reporter dyes. Using this technique, we were able to study the cellular uptake and distribution of fullerenes using a human mast cell (MC) model. It is shown that these fullerene derivatives are endocytosed into the cell and trafficked to different organelles at different time points. The fullerene conjugate localized with a particular predominance to calcium and ROS controlling organelles which may help explain how they exert their cellular inhibitory capabilities.
Methods
Fullerene derivatives
All fullerene derivatives were synthesized at Luna Innovations Incorporated. Texas Red (TR) was attached to the functionalized 70-carbon fullerene (C70), previously shown to demonstrate anti-inflammatory properties (3), as shown in Supplementary Figure 1. Monoprotected octa(ethyleneglycol)diamine [Supplementary Figure 1 - Step 1] (1 mmol) was reacted with ethylmalonylchloride (1 mmol) in the presence of triethylamine (TEA, 1.2 mmol) in anhydrous dichloromethane (DCM, 30 mL) for three hours at room temperature and the mixture was washed twice with brine before subjected to column chromatographic purification (silica gel). The isolated oily product [Supplementary Figure 1 - Step 2] was characterized by both 1H NMR and 13C NMR. Malonamide 2 (10 μmol) was reacted with one equivalent of iodine, 2.3 equivalents of 1,8-diazabicycloundec-7-ene (DBU), and 1.0 equivalent of the C70 monoadduct [Supplementary Figure 1 – Step 3] which was prepared from C70 and di(dodecyl)lmalonate under Bingel conditions (reaction to create extensions to the fullerene sphere). The reaction mixture was stirred for four hours in toluene, washed with brine, and concentrated for silica column purification. The isolated red band from silica column, characterized by both Nuclear magnetic resonance (NMR) and matrix assisted laser desorption ionization mass spectrometry (MALDI-MS), was treated with 20% trifluoroacetic acid (TFA) in DCM for two hours, and the solvent was rotavaped and pumped under vacuum for six hours. The resulting amine-functionalized C70 bisadduct [Supplementary Figure 1 – Step 4] (10 mg) in its TFA salt form was dissolved in 5 mL DMF and stirred with two equivalents of TEA for 10 minutes. Next, 2 mg of Texas Red NHS ester (Biotium, Inc.) dissolved in 0.2 mL amine-free DMF was added to the mixture and stirred for six hours at room temperature. DMF was removed under vacuum and the residue was subjected to preparative Thin layer chromatography (TLC - silica phase) for purification. After the TLC plate was spotted with the residue, it was eluted with a mixture of DCM and methanol. There were three bands: trace amounts of the dye on the top, product [Supplementary Figure 1 - Step 5] in the middle and unreacted C70 [Supplementary Figure 1 - Step 4] at the bottom. The plate was dried in a hood. The product band was scrapped off the plate, re-dissolved in a mixture of methanol and dimethylformamide (DCM, 1:1), filtered and concentrated to dryness, yielding pure 70-carbone fullerene-Texas Red (C70-TR) conjugate, which was characterized by MALDI-MS, UV-Vis and fluorescence spectrometry.
Human Mast Cell (MC) cultures and addition of fullerene derivatives
Human skin was received from the Cooperative Human Tissue Network. All studies were approved by their Human Studies Institutional Review Board. Tryptase-Chymase positive mast cells (MCTC) type (6) were purified and cultured as described (7–9). Mast cells were incubated with 5 μg/ml of C70-TR in normal medium for five hours, washed and photo-documented or placed back in culture for up to one week at 37°C in a 6% CO2 incubator. Preliminary experiments demonstrated that the C70-TR was maximally taken up within the MC at five hours; longer time points did not increase fullerene uptake. Thus, this time point was used in all subsequent experiments.
Endocytosis studies of fullerene derivatives
To determine the mechanisms of fullerene uptake MC were first pretreated with or without various inhibitors of cellular uptake (10 μg/mL each of filipin; inhibitor of the raft/caveolae endocytosis pathway, amiloride; inhibits clathrin-independent pathways, and indomethacin; which inhibits caveolae pathways). Cells were incubated for 30 minutes at 37°C in a humidified atmosphere with 6% CO2, washed and the fullerenes added for five hours before being washed and uptake monitored using confocal microscopy.
Confocal microscopy
Mast cells were incubated with C70-TR for five hours, washed, and incubated with the indicated organelle-specific dyes (mitotracker, lysotracker, and endoplasmic reticulum tracker; Invitrogen, CA) for the final 30 minutes. In some experiments, cells were treated as above and cytospins prepared at various times after washing. The MC-specific antibody, G3 (10), attached to FITC was used to examine secretory granule co-localization. Cells were visualized using a Yokogawa multiple lens Nipkow spinning disk scanner (CSU10) (Perkin Elmer Las, MA) illuminated by a ~100 mW Krypton/Argon laser mounted to a Nikon TE2000U inverted confocal microscope (Nikon Instruments Inc., NY). Images were captured using a Hamamatsu Orca ER (Hamamatsu Photonics, NJ) and SimplePCI software (Hamamatsu Corporation, PA).
Calcium and reactive oxygen species (ROS) measurement
Mast cells were pre-treated with or without C70-TR overnight, washed with Tyrodes buffer supplemented with BSA, and incubated with Fura-2 AM (2 μM; Invitrogen, CA) for 30 minutes at 37°C. Cells were washed, stimulated with an FcεRI activator (3B4), and calcium flux measured in real time on a Perkin Elmer LS55 Spectrofluorometer (Perkin Elmer Las, MA). For ROS production cells were exposed to fullerenes as above. After washing, cells were resuspended in normal medium containing 5 μM dichlorodihydrofluorescein (DCF) (2) at 37°C for 30 minutes, washed, and FcεRI activation-induced changes in mean fluorescence measured using spectroscopy with excitation at 502 nM and emissions at 523 nM for 15 minutes. The data are presented as fluorescence intensity of the 523 nM emission over time. All experiments were performed in triplicate and degranulation was measured in parallel. Separate experiments were performed to ensure that the fullerenes do not interfere with indicator dye binding (not shown).
Results
Design and synthesis of dye-conjugated fullerenes
A C70-based fullerene was selected for dye conjugation that is similar in structure to those determined to be potent anti-inflammatories and inhibitors of FcεRI-mediator release from MC ((3). The strategy for designing this C70-TR dye conjugate is shown step-wise in Supplemental Figure 1. Dye-conjugation to the fullerene compound was confirmed by MALDI-MS and absorption spectra (data not shown). All free, unconjugated dye was dialyzed to separate from the C70-TR conjugate.
Fullerenes enter MC through endocytosis
Little is known about how fullerenes enter cells and where fullerenes localize in situ. To examine the mechanisms of cellular entry we pre-incubated MC with several broad-acting endocytosis inhibitors and examined for fullerene localization within cells. In general, no fullerene localization was observed when cell were pre-treated with the endocytosis inhibitors filipin, amiloride (Figure 1). The use of other endocytosis inhibitors such as indomethacin demonstrated a similar lack of fullerene uptake which was not affected by higher fullerene concentrations or extended incubation times (not shown). Thus, fullerenes are taken up by human MC through an endocytosis pathway.
Figure 1. Endocytosis inhibitors block fullerene entry into cells.
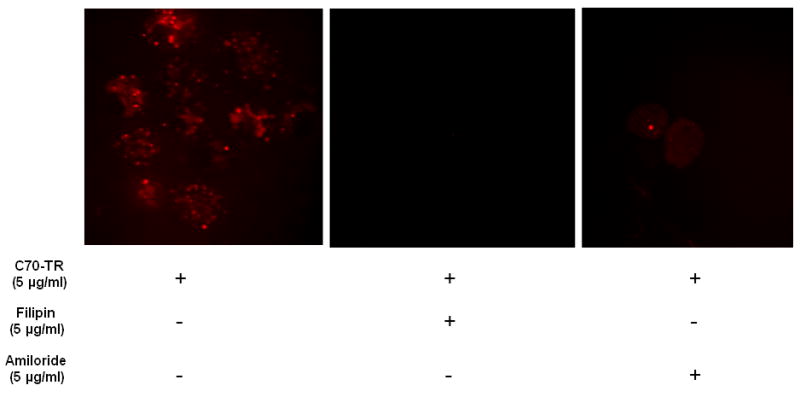
Skin MC were incubated with or without the indicated endocytosis inhibitors for 16 hours in the tissue culture incubator. The next day cells were incubated with the C70-TR for 5 hours, washed, and analyzed by confocal microscopy. Pictures (63x oil; cropped as needed) representative of two separate experiments.
Intracellular trafficking of C70-TR
Time-course studies were performed to determine the localization of the fullerenes in human MC. As seen in Figure 2A–2F the majority of the C70-TR was observed in the endolasmic reticulum and lysosomes at four hours post wash out (nine hours total). Minimal colocalization was observed within the mitochondria (Figure 2G–2I) at this time-point. Similar localization was observed at 24 and 96 hours post wash-out with less co-localization within the lysosomes (Figures 3A–4F and 4A–4F). The fullerenes were detected up to one week within the cells although there was notably less C70-TR within the cell (Figure 5A–5F). As with earlier time-points there was a predominance of fullerene localization with the ER. The presence of the fullerenes in the cells did not affect cell viability at any of the times examined which was consistently >90% and not significantly different compared to non-treated cells (not shown). This suggests fullerenes are not quickly shuttled out of the cell but persist within organelles involved in FcεRI-dependent mediator release.
Figures 2–5. Time course of organelle localization using C70-TR in human MC.
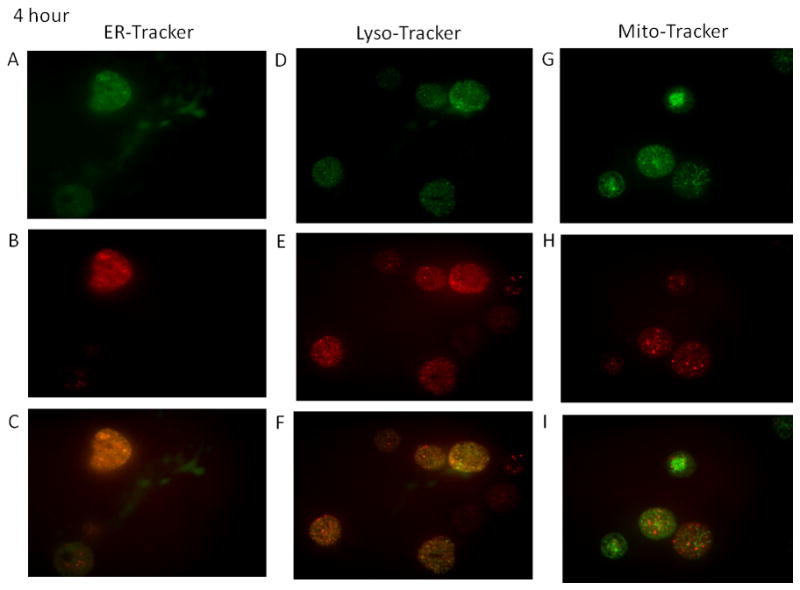
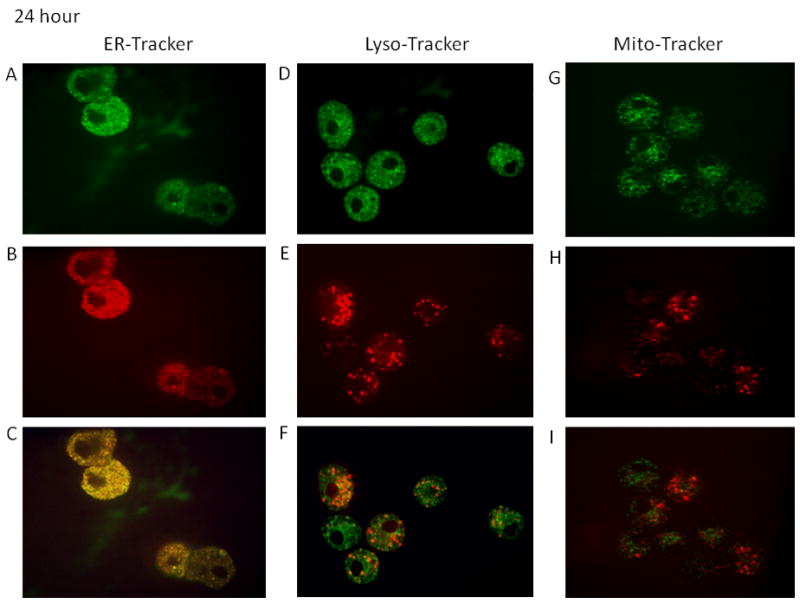
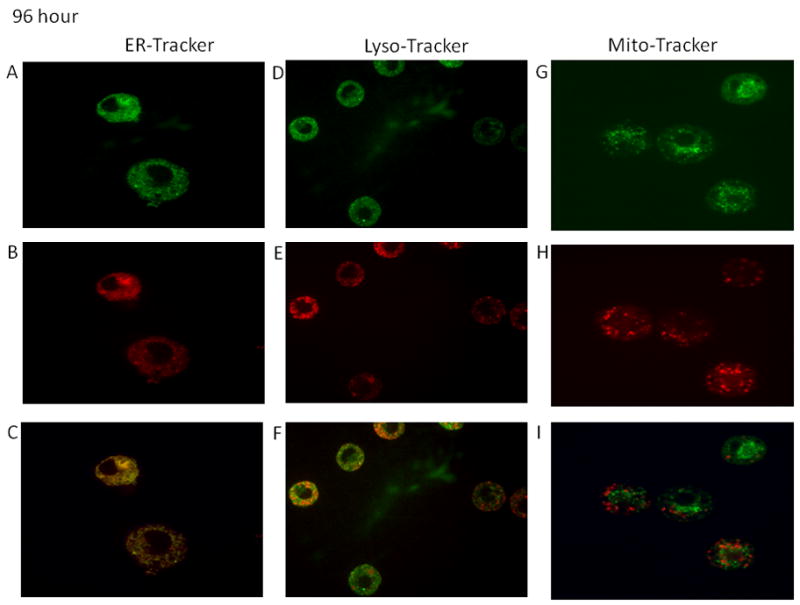
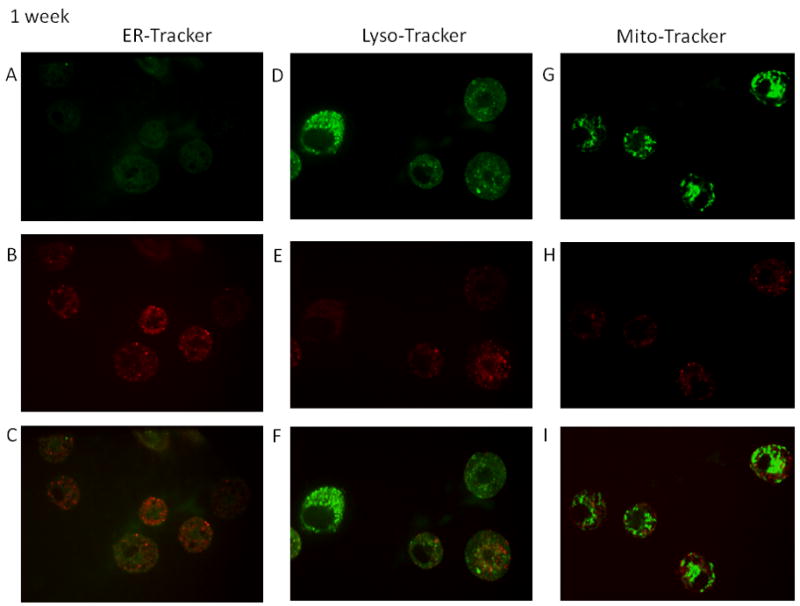
Cells were incubated with Texas Red conjugated-C70 fullerenes (5 μg/ml) for 5 hours, washed, and placed back in culture for the indicated times. The final 30 min cells were incubated with ERtracker (500 nM; A–C), lysotracker (25 nM; D–E), or mitotracker (20 nM; F–H), washed, placed on a coverslip, and imaged using confocal microscopy. Pictures (63x oil; cropped as needed) representative of two separate MC culture.
C70-TR fullerene colocalized in ER reduces calcium release and ROS production
The activation of MC FcεRI leading to degranulation is calcium dependent and induces elevated cellular levels of reactive oxygen species (ROS) (11;12). Given that calcium and ROS are produced within ER and mitochondria and we and others have demonstrated that these organelles accumulate fullerenes with varying affinities, we hypothesized that the co-localization of fullerene derivatives developed herein would inhibit these cellular mediators. The intracellular calcium and ROS levels in FcεRI-challenged cells with or without pre-incubation with fullerenes were measured to determine the impact that this fullerene has on both calcium stores and ROS levels. As seen in Figure 6, the C70-TR inhibited calcium (Figure 6A and 6B) and ROS levels (Figure 6C and 6D) as compared to the untreated MC control. In general, a higher inhibition of release of intracellular calcium stores occurred compared to ROS levels.
Figure 6. C70-TR fullerene colocalized in ER reduces calcium release and ROS production.
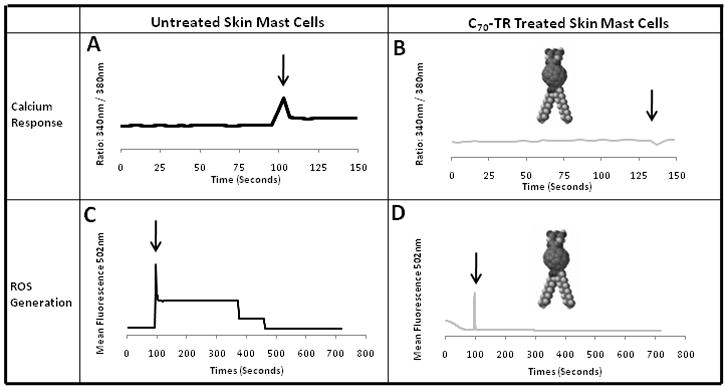
FcεRI stimulation (3B4; 1 μg/ml) of fullerene treated cell (20 μg/ml) were examined in parallel for calcium stores release as determined by the 340/380 nm ratio and ROS measured by DCF detection. Results show average value (±S.D.) from at least 3 separate experiments and donors. Experiment represents three separate MC preparations.
Discussion
These are the first studies examining fullerene trafficking within human MC. It was observed that the C70-TR conjugated fullerenes were taken up through an endocytosis-dependent mechanism and persisted in the MC for up to one week. The intracellular localization was found predominately in the ER and to a lesser degree in mitochondria and lysosomes. No fullerenes were detected in the tryptase-containing secretory granules or in the nucleus at any time-points measured. Taken together, it is clear that MC actively take up fullerenes through an endocytotic pathway where they remain mostly localized to ER and to a lesser extent in lysosomes and mitochondria.
The ER is the site for synthesizing and ensuring proper folding of proteins within a cell and shuttles misfolded proteins through a degradative pathway. Recent evidence suggests this process involves the formation of disulfide bonds that stabilize the folding of nascent proteins resulting in an oxidizing environment and ROS generation (13). In addition to the ER, mitochondria also produce ROS due to the production of ATP following oxidative phosphorylation. Our results using C70-based derivatives are consistent with several publications examining fullerene localization in different cell types in which deposition in different intracellular organelles depends on the moieties added to the carbon cage (2;14–17). For example, C63(COOH)6 or C61(CO2H)2 appear to localize to mitochondria (14;16) while C60 mixtures dispersed in tetrahydrofuran (and not purified from this solvent) localize to lysosomes and nuclei in macrophages (17). Our results are also consistent with previously studies suggesting fullerenes can easily penetrate into a lipid membrane where they induce non-toxic changes in the structural and elastic properties of the lipid bilayer at very high concentrations (18). Taken together, fullerenes as a general class have a varied localization profile within cells; how the fullerene cage is modified determines where the cage localizes in situ. Given that no fullerene formulation has yet been identified for treating a particular disease it is difficult to extrapolate in situ localization results from different fullerene preparations.
The results demonstrating that C70-based fullerenes are endocytosed and localize to ER differentiate from previously published results showing that endocytosed C60-based fullerenes localize to the mitochondria and lysosomes (19;20). The ER accumulation of C70-based fullerenes explains the data shown that pre-incubation of MC with the same fullerenes caused a reduction of FCεRI-mediated calcium release and ROS generation. These findings may help explain fullerenes mechanism of inhibition of FcεRI activated mediator release from MC. In a separate publication (J. Immunology.) we demonstrate that the C70-based derivatives not unlike the one used for the TR conjugation blunts histamine degranulation and cytokine release when MC are pre-challenged with equivalent concentrations and times as described herein. The release of calcium stores and production of ROS occur in these organelles (21); while release of calcium stores is absolutely required for FcεRI-mediator release it is still no clear if increased ROS levels parallel mediator release or is a consequence of FcεRI crosslinking. Calcium stores release from the ER in response to FcεRI crosslinking is controlled by inositol 1,4,5-trisphosphate. Thus, the observation that fullerenes localize to this organelle fits well with our data showing that calcium stores release and ROS production are inhibited when FcεRI-challenged MC are pre-challenged with fullerenes. Current efforts are aimed at determining what ER-associated signaling molecules (if any) are bound to fullerenes which would help explain how these molecules exert their inhibitory activities.
Fullerenes were also consistently found (but to a lesser extent as compared to ER accumulation) in lysosomes. Endocytosis from the plasma membrane can occur by a variety of mechanisms and once uptake occurs the molecules are then routed through lysosomes to various places within cells. We observed lysosomal accumulation of C70-TR predominately at four hour, 96 hour, and one week after washout perhaps indicating the cells shuffling the conjugates from the membrane early and to the membrane for possible excretion at the later times. Indeed, there appears to be a localization of fullerenes in the outer edges of the cell at later times (Figures 4 and 5) possibly indicated that their imminent transport out of the cells.
As mentioned above, the mitochondria is another organelle that is best known for its role in ROS production which is a result of the electrochemical membrane potential (ΔΨm) across the inner mitochondrial membrane (21). We found sporadic localization of the C70-TR in the mitochondria at some of the times examined. Mitochodrial localization has been demonstrated previously either directly or indirectly using other fullerene preparations (14;16;22;23). Given that fullerenes are potent anti-oxidants that can react with ROS it has been speculated that much of their inhibitory capabilities are linked to this radical scavenging ability. However, we show here that fullerenes that are effective anti-inflammatory agents mainly target ER which is also a ROS-producing organelle. It is possible the C70-TR may not reflect the same targeting specificity as the C70 derivative without TR. We are currently engineering fullerenes without bulky side chains that are structurally the same as those previously described so that results obtained on inhibitory capabilities can be extrapolated to organelle targeting more specifically.
In conclusion, we have identified the ER as a primary organelle for 70-carbon based fullerene derivatives localization in human MC as opposed to previous publications which show 60-carbon based fullerenes localize to the mitochondria This localization may help explain how the fullerenes exhibit their inhibitory activity through blunting of calcium and ROS spikes leading to subsequent reduction in histamine deganulation and cytokine production. Fullerenes are potent antioxidants and are being investigated as therapies for a wide range of diseases (24–26). This may have clinical implications for developing future fullerene-based compounds, our efforts are focused on developing these lead candidates into novel ways to treat those diseases associated with MC activation including asthma, arthritis, and anaphylaxis.
Supplementary Material
Synthesis of fullerene-Texas Red conjugates. C70 was sequentially derivatized at the two poles with one lipophilic and one hydrophilic group. Texas Red was covalently conjugated to the polar pole of C70 through a short polyethylene glycol (PEG7) spacer via an amide bond.
Acknowledgments
This work was supported NIH Grants 1R01GM083274-01 and 1R43HL087578-01A1 (CLK). D.H.C. acknowledges NIH Grants R01AI018697-28 and U19AI077435 subproject #2. The confocal microscopy was performed at the Hooker Imaging Center at the University of North Carolina.
Abbreviations
- MC
Mast cell
- MCTC
Tryptase-Chymase positive mast cell
- ER
Endoplasmic reticulum
- ATP
Adenosine tri-phosphate
- TR
Texas red
- FITC
Fluorescein isothiocyanate
- C70
70-Carbon fullerene
- ROS
Reactive oxygen species
- NMR
Nuclear magnetic resonance
- DBU
1,8-Diazabicycloundec-7-ene
- TFA
Trifluoroacetic acid
- DCM
Dichloromethane
- DMF
Dimethylformamide
- TEA
Triethylamine
- MALDI-MS
Matrix assisted laser desorption ionization mass spectrometry
- TLC
Thin layer chromatography
- DCF
Dichlorodihydrofluorescein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson SR, Schuster DI, Nuber B, Meier M, Prato M, Taylor R. In: Fullerenes: Chemistry, Physics, and Technology. Kadish K, Ruoff R, editors. John Wiley & Sons; NY: 2000. [Google Scholar]
- 2.Ryan JJ, Bateman HR, Stover A, Gomez G, Norton SK, Zhao W, et al. Fullerene nanomaterials inhibit the allergic response. J Immunol. 2007;179(1):665–72. doi: 10.4049/jimmunol.179.1.665. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger A, Zhou Z, Lenk R, Macfarland D, Kepley CL. Fullerene nanomaterials inhibit phorbol myristate acetate-induced inflammation. Exp Dermatol. 2009 doi: 10.1111/j.1600-0625.2009.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dugan LL, Turetsky DM, Du C, Lobner D, Wheeler M, Almli CR, et al. Carboxyfullerenes as neuroprotective agents. Proc Natl Acad Sci U S A. 1997;19;94(17):9434–9. doi: 10.1073/pnas.94.17.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Z, Lenk R, Dellinger A, Macfarland D, Kumar K, Wilson SR, et al. Fullerene nanomaterials potentiate hair growth. Nanomedicine. 2009 doi: 10.1016/j.nano.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz LB, Kepley C. Development of markers for human basophils and mast cells. J Allergy Clin Immunol. 1994;94:1231–40. doi: 10.1016/0091-6749(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 7.Kepley CL, Cohen N. Evidence for human mast cell nonreleaser phenotype. J Allergy Clin Immunol. 2003;112(2):457–9. doi: 10.1067/mai.2003.1671. [DOI] [PubMed] [Google Scholar]
- 8.Kepley CL. Antigen-induced reduction in mast cell and basophil functional responses due to reduced Syk protein levels. Int Arch Allergy Immunol. 2005;138(1):29–39. doi: 10.1159/000087355. [DOI] [PubMed] [Google Scholar]
- 9.Kepley CL, Youssef L, Andrews RP, Wilson BS, Oliver JM. Multiple defects in Fc epsilon RI signaling in Syk-deficient nonreleaser basophils and IL-3-induced recovery of Syk expression and secretion. J Immunol JID - 2985117R. 2000;165(10):5913–20. doi: 10.4049/jimmunol.165.10.5913. [DOI] [PubMed] [Google Scholar]
- 10.Irani A-MA, Bradford TR, Kepley CL, Schechter NM, Schwartz LB. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem. 1989;37:1509–15. doi: 10.1177/37.10.2674273. [DOI] [PubMed] [Google Scholar]
- 11.Sly LM, Kalesnikoff J, Lam V, Wong D, Song C, Omeis S, et al. IgE-induced mast cell survival requires the prolonged generation of reactive oxygen species. J Immunol. 2008;181(6):3850–60. doi: 10.4049/jimmunol.181.6.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swindle EJ, Metcalfe DD. The role of reactive oxygen species and nitric oxide in mast cell-dependent inflammatory processes. Immunol Rev. 2007;217:186–205. 186–205. doi: 10.1111/j.1600-065X.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu Y, Hendershot LM. Organization of the functions and components of the endoplasmic reticulum. Adv Exp Med Biol. 2007;594:37–46. doi: 10.1007/978-0-387-39975-1_4. [DOI] [PubMed] [Google Scholar]
- 14.Chirico F, Fumelli C, Marconi A, Tinari A, Straface E, Malorni W, et al. Carboxyfullerenes localize within mitochondria and prevent the UVB-induced intrinsic apoptotic pathway. Exp Dermatol. 2007;16(5):429–36. doi: 10.1111/j.1600-0625.2007.00545.x. [DOI] [PubMed] [Google Scholar]
- 15.Salonen E, Lin S, Reid ML, Allegood M, Wang X, Rao AM, et al. Real-time translocation of fullerene reveals cell contraction. Small. 2008;4(11):1986–92. doi: 10.1002/smll.200701279. [DOI] [PubMed] [Google Scholar]
- 16.Foley S, Crowley C, Smaihi M, Bonfils C, Erlanger BF, Seta P, et al. Cellular localisation of a water-soluble fullerene derivative. Biochem Biophys Res Commun. 2002;294(1):116–9. doi: 10.1016/S0006-291X(02)00445-X. [DOI] [PubMed] [Google Scholar]
- 17.Porter AE, Muller K, Skepper J, Midgley P, Welland M. Uptake of C60 by human monocyte macrophages, its localization and implications for toxicity: studied by high resolution electron microscopy and electron tomography. Acta Biomater. 2006;2(4):409–19. doi: 10.1016/j.actbio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Wong-Ekkabut J, Baoukina S, Triampo W, Tang IM, Tieleman DP, Monticelli L. Computer simulation study of fullerene translocation through lipid membranes. Nat Nanotechnol. 2008;3(6):363–8. doi: 10.1038/nnano.2008.130. [DOI] [PubMed] [Google Scholar]
- 19.Sumizawa T, Igisu H. Suppression of acrylamide toxicity by carboxyfullerene in human neuroblastoma cells in vitro. Arch Toxicol. 2009;83(9):817–24. doi: 10.1007/s00204-009-0438-7. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Chen C, Ye C, Wei T, Zhao Y, Lao F, et al. The translocation of fullerenic nanoparticles into lysosome via the pathway of clathrin-mediated endocytosis. Nanotechnology. 2008;19(14):145102. doi: 10.1088/0957-4484/19/14/145102. [DOI] [PubMed] [Google Scholar]
- 21.Feissner RF, Skalska J, Gaum WE, Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci. 2009;14:1197–218. doi: 10.2741/3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monti D, Moretti L, Salvioli S, Straface E, Malorni W, Pellicciari R, et al. C60 carboxyfullerene exerts a protective activity against oxidative stress-induced apoptosis in human peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2000;277(3):711–7. doi: 10.1006/bbrc.2000.3715. [DOI] [PubMed] [Google Scholar]
- 23.Fumelli C, Marconi A, Salvioli S, Straface E, Malorni W, Offidani AM, et al. Carboxyfullerenes protect human keratinocytes from ultraviolet-B-induced apoptosis. J Invest Dermatol. 2000;115(5):835–41. doi: 10.1046/j.1523-1747.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- 24.Bakry R, Vallant RM, Najam-ul-Haq M, Rainer M, Szabo Z, Huck CW, et al. Medicinal applications of fullerenes. Int J Nanomedicine. 2007;2(4):639–49. [PMC free article] [PubMed] [Google Scholar]
- 25.Djordjevic A, Bogdanovic G, Dobric S. Fullerenes in biomedicine. J BUON. 2006;11(4):391–404. [PubMed] [Google Scholar]
- 26.Jensen AW, Wilson SR, Schuster DI. Biological applications of fullerenes. Bioorg Med Chem. 1996;4(6):767–79. doi: 10.1016/0968-0896(96)00081-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthesis of fullerene-Texas Red conjugates. C70 was sequentially derivatized at the two poles with one lipophilic and one hydrophilic group. Texas Red was covalently conjugated to the polar pole of C70 through a short polyethylene glycol (PEG7) spacer via an amide bond.


