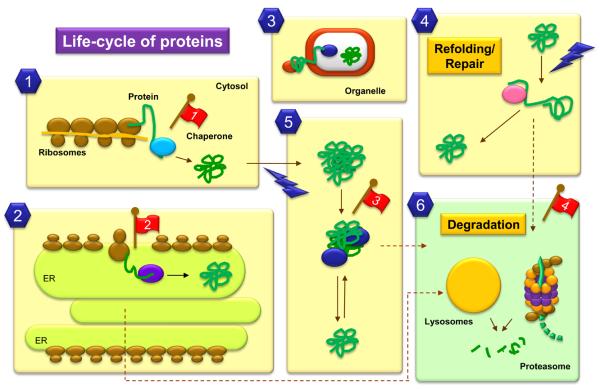
Figure 1. Cellular events that involve protein folding, refolding and degradation.
Most cytosolic proteins fold spontaneously after synthesis, but those that fail to acquire a proper folded conformation are aided by chaperones and chaperonins which provide a favorable folding environment (1). Chaperones also assist folding of proteins synthesized in the endoplasmic reticulum (ER) (2). Further failure to fold will destine both cytosolic and ER luminal proteins for degradation (6). Unfolding of previously folded protein is required for their trafficking across membranes (3) and for their assembly into protein complexes. Chaperones also assist these proteins in their refolding after such events. Lastly, proteins can be targets for damaging agents (4 and 5) which lead to their unfolding and/or their aggregation. Chaperones will assist in refolding (4) or disaggregation (5). However, when the damage is irreversible molecular chaperones mediate the degradation of the altered proteins by the different proteolytic systems (6). Red flags indicate some steps altered with aging: 1. Reduced content of cytosolic chaperones; 2. Impaired ER response to stress; 3. Increased content of aggregated proteins; 4. Deficient targeting of damaged proteins toward degradation.