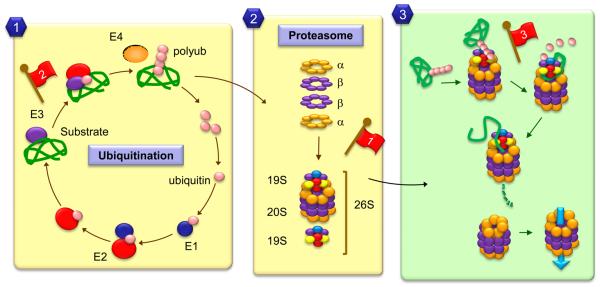
Figure 2. Schematic model of the ubiquitin/proteasome system.
The main events that mediate targeting and degradation of soluble proteins by the proteasome are depicted here. 1. Most proteins are targeted for degradation through the covalent attachment of 4-5 ubiquitins through a lysine residue in their sequence. Ubiquitinization requires the coordinated action of catalytic enzymes (E1, E2 and E3) that act sequentially to activate the ubiquitin and ligate it to the substrate presented by the E3. 2. The proteolytic component, the proteasome or 26S, has a catalytic core (the 20S) formed by 4 rings containing two types of catalytic subunits (α and β), and a regulatory complex (the 19S). 3. Polyubiquitin chains are recognized by components of the regulatory subunit, where deubiquitinases reverse the covalent conjugation releasing free ubiquitin for recycling. The substrate is unfolded by unfoldases in the regulatory lid and ATPases in this complex provide the energy required for the injection of the substrate protein into the catalytic barrel or 20S proteasome. Red flags indicate described age-related changes in the different steps of this process: 1. Lower levels of catalytic and/or regulatory subunits and inefficient assembling of the 26S proteasome; 2. Reduced content/activity of free ubiquitin and conjugating enzymes; 3. Posttranslational modifications and crosslinking of the substrates can interfere with proteasome activity.