SUMMARY
Objective
Adipose tissue-derived inflammation may contribute to metabolic alterations and eventually to the metabolic syndrome (MetS). The purpose of this study was to: 1) examine the role of adipocytokines in the association between obesity and the MetS; and 2) to determine whether the association is different in obese and non-obese persons.
Design
Cross-sectional population-based InCHIANTI study.
Subjects
944 community-dwelling adults aged 65 years and older living in Tuscany, Italy.
Measurements
Obesity was defined as body mass index ≥ 30 kg/m2 and MetS as ≥ 3 of the ATP-III criteria. Circulating levels of CRP, IL-6, IL-1ra, IL-18, TNF-α R1, adiponectin, resistin, and leptin were measured. Additionally, insulin resistance was determined using the homeostasis model assessment (HOMA-IR).
Results
The prevalence of the MetS was 32%. Both overall and abdominal obesity were significantly associated with the MetS after adjusting for inflammatory cytokines, adipokines and lifestyle factors. After adjusting for multiple confounders and HOMA-IR, IL-1ra, TNF-α R1 and adiponectin (p < 0.05) remained significantly associated with the MetS. Having multiple cytokines in the highest tertile increased the likelihood of having the MetS in both obese (p for trend 0.002) and non-obese persons (p for trend 0.001) independent of insulin resistance.
Conclusions
Non-obese and obese individuals who develop an intense pro-inflammatory state may be more prone to develop the MetS than those with lower levels of inflammation.
Keywords: adipocytokines, adiponectin, cytokines, inflammation, metabolic syndrome, obesity
INTRODUCTION
Obesity is a known risk factor for diseases, functional decline, disability, and mortality 1. However, because obesity is typically associated with other risk factors, such as a sedentary lifestyle, high levels of circulating lipids and hypertension, the pathway by which obesity independently affect these negative health outcomes is not clear.
Although the prevalence of the metabolic syndrome (MetS) is higher among obese persons, not all obese individuals display a clustering of metabolic and cardiovascular risk factors 2, 3. Furthermore, characteristic features of the MetS are also found in lean individuals 3, 4. The mechanisms by which the MetS develops in certain individuals and not in others are not understood. Adipose tissue, especially in the visceral area, is an active endocrine organ that produces various inflammatory cytokines, such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α, and adipokines, such as leptin and adiponectin 5, 6. An inflammatory state may lead to individual metabolic alterations that result in the features of the MetS 7. Therefore, obese individuals who develop an intense pro-inflammatory state may be more likely to develop the MetS than those with lower levels of inflammation.
The purpose of this study was to: 1) examine the role of inflammatory adipocytokines in the association between obesity and the MetS; and 2) to determine whether the association is different in obese and non-obese older persons.
METHODS
Study population
The InCHIANTI Study (Invecchiare in Chianti, aging in the Chianti area) is an epidemiological study of a representative sample of the population living in Tuscany, Italy. Overall, 1260 persons aged 65 years and older were randomly selected from the population registry and were eligible for the study. 1155 older adults agreed to participate in the study (participation rate 91.7%) and 1055 donated a blood sample. Of these, 944 had data on body mass index (BMI), waist circumference, metabolic syndrome and adipocytokines and were included in this study. InCHIANTI design and data collection have been described in detail 8. Participants received an extensive description of the study and provided written informed consent. The Italian National Institute of Research and Care on Aging Ethical Committee approved the study protocol, which complied with the principles of the Declaration of Helsinki.
Measures
Anthropometrics
BMI was calculated as weight (kg) divided by height squared (m2). Weight was measured to the nearest 0.1 kg using a high-precision mechanical scale and standing height to the nearest 0.1 cm based on wall measure with participants wearing light indoor clothes and no shoes. Obesity was defined as BMI ≥ 30 kg/m2 9. Underweight (BMI < 18.5 kg/m2) participants were excluded from the analyses (n = 4).
Waist circumference was measured at the midpoint between the lower rib margin and the iliac crest and was categorized as: 1) Normal < 80 cm in women and < 94 cm in men; 2) Increased 80–87 cm in women and 94–101 cm in men; or 3) Large ≥ 88 cm in women and ≥ 102 cm in men 9.
Metabolic alterations
In accordance with the National Cholesterol Education Program Adult Treatment Panel III criteria (NCEP ATP-III) 10, MetS was defined as the presence of three or more of the following five features: 1) waist circumference ≥ 102 cm in men and ≥ 88 cm in women; 2) fasting serum triglycerides of ≥ 150 mg/dL or lipid-lowering treatment; 3) fasting serum high-density lipoprotein (HDL) cholesterol < 40 mg/dL in men and < 50mg/dL in women; 4) systolic blood pressure ≥ 130 and/or diastolic blood pressure ≥ 85 mgHg or antihypertensive treatment; 5) fasting serum glucose levels of ≥ 100 mg/dL or anti-diabetic treatment. Blood pressure was recorded using a standard mercury sphygmomanometer. All blood pressure measurements were performed three times with the participant in a supine position separated by intervals of two minutes; the average of the last two measures was used in the analysis.
Laboratory measurements
Fasting blood samples were collected in the morning after a 12-hour fast and after a 15-minute rest. Serum and plasma were stored at −80°C until analysis.
Metabolic determinants
Fasting serum glucose level was determined using an enzymatic colorimetric assay (Roche Diagnostics, Mannheim, Germany) and a Roche-Hitachi 917 analyzer. A commercial enzymatic test was used for determining serum HDL cholesterol and triglyceride concentrations (Roche Diagnostics). The inter-assay coefficient of variation (CV) was less than 3.8% for HDL cholesterol and less than 2.5% for triglycerides. Fasting insulin was determined using a commercial double-antibody, solid-phase radioimmunoassay (Sorin Biomedica, Milan, Italy) with an intra-assay coefficient of 3.1%. Using data on fasting glucose and insulin, the degree of insulin resistance was calculated according to the homeostasis model assessment (HOMA-IR), which is a good index for assessing insulin resistance across a wide range of values and is well correlated with insulin-mediated glucose uptake calculated by euglycemic glucose clamp 11, 12.
Adipocytokines
Biomarkers were chosen for the present study because of their association with obesity or the MetS 13-15. High sensitivity C-reactive protein (CRP) levels were measured by enzyme-linked immunoabsorbent assay (ELISA) using purified protein and polyclonal anti-CRP antibodies (Roche Diagnostics, GmbH, Mannheim, Germany). For high sensitivity CRP the minimum detectable concentration (MDC) was 0.03 mg/L and the inter-assay CV was 5%. Serum IL-6 (MDC=0.1 pg/mL; CV=4.5%), interleukin 1 receptor antagonist (IL-1ra) (MDC=4.00 pg/mL; CV=8.2%.) and TNF-α receptor 1 (TNF-α R1) (MDC≈8 pg/mL; CV=10%) concentrations were determined by high-sensitive ELISA using commercial kits (Human Ultrasensitive, BioSource International Inc., Camarillo, CA, USA). IL-1ra and TNF-α R1 were used instead of IL-1 and TNF-α because measurements of soluble receptors are more sensitive and easier than those for the respective cytokines 16, 17. IL-18 concentration was measured by sandwich ELISA (Human IL-18 ELISA Kit, Medical and Biological Laboratories Co, Ltd, Nagoya, Japan) (MDC=12.5 pg/mL; CV < 10.8%). Serum adiponectin concentration was measured using RIA assay (Human Adiponectin RIA Kit; LINCO Research, Inc, MO, USA) (MDC=1 ng/mL in 100 μL sample; CV < 10%). Serum leptin was determined using ELISA (Human Endocrine LINCOplex Kit, LINCO Research, Inc., St. Charles, MO, USA) (MDC=1 ng/mL in 100 μL sample; CV < 7%). Serum resistin concentration was measured using ELISA assay (Alpco Diagnostics, Salem, NH, USA) (MDC=0.2 ng/mL; CV<7%). All cytokine assays were done in duplicate for adipocytokine measures and were repeated if the second measure was >10% or <10% compared to the first. The average of the two measures was used in the analyses.
For each biomarker, values were divided into tertiles based on their distribution. For CRP, IL-6, IL-1ra, IL-18 and TNF-α R1, the highest tertile was considered indicative of cytokine dysregulation. Adipokine dysregulation was indicated by the highest tertile for leptin and resistin and by the lowest tertile for adiponectin. To examine the combined effect of dysregulated cytokines and adipokines the total number of biomarkers in the highest (lowest for adiponectin) tertile was calculated. The cut-off values for the highest biomarker tertiles were the following: CRP 4.27 mg/L, IL-6 1.84 pg/mL, IL-1ra 164.39 pg/mL, IL-18 435.32 pg/mL and TNF-α R1 1546.20 pg/mL, resistin 4.30 ng/mL, leptin 13.50 ng/mL, adiponectin 80.10 ng/mL (lowest tertile)
Covariates
The level of physical activity in the 12 months prior to the interview was assessed through an interviewer-administered questionnaire and was categorized as: 1) sedentary / minimal physical activity; 2) light physical activity performed 2–4 hours per week not accompanied by sweating; 3) moderate physical activity performed 1–2 hours per week accompanied by sweating or light physical activity not accompanied by sweating for ≥ 4 hours per week; or 4) moderate / intense physical activity performed for ≥ 3 hours per week accompanied by sweating. Based on a smoking history questionnaire, subjects were categorized as never smokers, former smokers or current smokers. Daily alcohol intake (g) was estimated by the European Prospective Investigation into Cancer and Nutrition Food Frequency Questionnaire 18. Presence of coronary heart disease, peripheral arterial disease, type 2 diabetes, lung disease and osteoarthritis was ascertained by a trained geriatrician according to standard, pre-established criteria and algorithms, based on those used in the Women’s Health and Aging Study, that combine information from self-reported physician diagnoses, current pharmacological treatment, medical records, clinical examinations and blood tests 19.
Statistical analysis
Study population characteristics according to the MetS status are reported as mean values and standard deviations for continuous variables and proportions for categorical variables. Comparisons across groups were examined with Chi-square test for categorical variables, Kruskal-Wallis test for skewed continuous variables and t-test for normally distributed continuous variables. Correlations between metabolic determinants (continuous variables) and adipocytokines were examined with Spearman correlation coefficients. Additionally, collinearity between adipocytokines was examined using variance inflation factors in regression models. All variance inflation factors were below 10, indicating non-significant multicollinearity. Thus, all the adipocytokines were entered simultaneously in the logistic regression models.
To examine the role of adipocytokines on the association between obesity and MetS, a series of logistic regression models were constructed. Both BMI and waist circumference were used as independent variables in predicting the MetS. Model 1 included BMI, age and sex. Model 2 included adipocytokines, age and sex. Model 3 included BMI, age, sex and adipocytokines. Model 4 included BMI, adipocytokines, age, sex, education, physical activity, smoking and alcohol consumption. Due to non-normally distributed adipocytokine values, biomarkers were log transformed (natural logarithm - Ln) to approximate a normal distribution. To allow for a direct comparison between adipocytokines, standardized odds ratios are reportedindicating odds per standard deviation increment in adipocytokine. Because the definition of the MetS includes waist circumference, in models examining the association between waist circumference and MetS (Table 4), we eliminated waist circumference from the ATP-III criteria for the MetS and participants were considered to have MetS if they met three of four criteria 20.
Table 4.
Odds ratios for the metabolic syndrome associated with waist circumference and the mediating role of adipocytokines *
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Waist circumference † | ||||||||
| Normal (ref) (n=277) | 1 | 1 | 1 | |||||
| Increased (n=285) | 2.10 | 1.23–3.58 | 1.58 | 0.82–3.05 | 1.45 | 0.74–2.85 | ||
| Large (n=382) | 4.34 | 2.60–7.26 | 3.25 | 1.64–6.44 | 2.89 | 1.42–5.86 | ||
| Cytokines ‡ | ||||||||
| Log CRP (mg/L) | 1.00 | 0.78–1.28 | 0.95 | 0.74–1.22 | 0.87 | 0.67–1.14 | ||
| Log IL-6 (pg/mL) | 1.07 | 0.83–1.36 | 1.08 | 0.84–1.39 | 1.01 | 0.77–1.31 | ||
| Log IL-1ra (pg/mL) | 1.42 | 1.08–1.86 | 1.40 | 1.07–1.83 | 1.73 | 1.28–2.33 | ||
| Log IL-18 (pg/mL) | 1.21 | 0.96–1.52 | 1.21 | 0.96–1.53 | 1.22 | 0.96–1.56 | ||
| Log TNF-α R1 (pg/mL) | 1.21 | 0.95–1.55 | 1.21 | 0.95–1.56 | 1.17 | 0.90–1.51 | ||
| Adipokines ‡ | ||||||||
| Log Adiponectin (ng/mL) | 0.50 | 0.40–0.62 | 0.50 | 0.41–0.63 | 0.49 | 0.39–0.62 | ||
| Log Leptin (ng/mL) | 1.42 | 1.08–1.86 | 1.14 | 0.85–1.54 | 1.08 | 0.79–1.48 | ||
| Log Resistin (ng/mL) | 1.04 | 0.83–1.30 | 1.05 | 0.84–1.32 | 1.01 | 0.79–1.29 | ||
Metabolic syndrome defined without waist circumference component.
Normal < 80 cm in women and < 94 cm in men; Increased 80–87cm in women and 94–101 cm in men; Large ≥ 88 cm in women and ≥ 102 cm in men.
Adipocytokines are presented as standardized values and OR indicates odds per standard deviation (SD) increment in adipocytokine.
Model 1 included BMI, age and sex. Model 2 included adipocytokines, age and sex. Model 3 included BMI, adipocytokines, age and sex. Model 4 included BMI, adipocytokines, age, sex, education, physical activity, smoking and alcohol consumption.
OR = odds ratio; CI = confidence interval; CRP = C-reactive protein (standard deviation SD=1.05 mg/L); IL-6 = interleukin 6 (SD=0.84 pg/mL); IL-1ra = interleukin 1 receptor antagonist (SD=0.57 pg/mL); IL-18 = interleukin 18 (SD=0.35 pg/mL), TNF-α R1 = tumor necrosis factor- α receptor 1 (SD=0.35 pg/mL). Adiponectin SD=0.73 ng/mL; Leptin SD=1.04 ng/mL; Resistin SD=0.45 ng/mL.
Finally, we examined whether the association between adipocytokines and the MetS is different among obese and non-obese persons. The age-adjusted prevalence of the MetS according to number of dysregulated cytokines and adipokines was examined. Further, logistic regression analyses were performed to examine the likelihood of the MetS according to the levels of the individual adipocytokines as well as to the number of dysregulated cytokines and adipokines. Linear trends for dysregulated cytokines and adipokines were calculated using generalized linear models (GLM) by entering categorical variables in the model as ordinal variables. Because one common denominator underlying the relationship between obesity and the MetS is likely to be insulin resistance, models were also adjusted also for HOMA-IR value.
Three-way interactions of sex, obesity and different adipocytokines on the MetS were tested using GLM. Because they were all non-significant, men and women were pooled together for analysis. The SAS 9.1 Statistical Package was used for all analyses (SAS Institute, Inc., Cary, North Carolina, USA).
RESULTS
The prevalence of the MetS in the study population was 32.2% and was lower in men (24.1%) than in women (38.7%) (p < 0.001). The prevalence of the MetS was 59.3% among obese (BMI ≥ 30 kg/m2) and 23.2% among non-obese participants. Other characteristics of the study population are shown in Table 1.
Table 1.
Characteristics of the study population according to the presence of the metabolic syndrome
| All (n=944) |
No MetS (n=640) | MetS (n=304) |
p value* | |
|---|---|---|---|---|
| Age (years), mean (SD) | 74.3 (6.8) | 74.3 (6.9) | 74.4 (6.6) | 0.77 |
| Men, % | 44.49 | 49.84 | 33.22 | <.001 |
| Education (years) mean (SD) | 5.4 (3.2) | 5.6 (3.4) | 5.0 (2.8) | 0.005 |
| Body Mass Index ≥ 30 kg/m2, % | 25.0 | 15.0 | 46.1 | <.001 |
| Physical activity, % | <.001 | |||
| Sedentary / minimal | 17.22 | 14.91 | 22.04 | |
| Light | 44.31 | 42.07 | 49.01 | |
| Moderate | 32.94 | 36.26 | 25.99 | |
| Intense | 5.53 | 6.75 | 2.96 | |
| Smoking, % | 0.13 | |||
| Never | 58.47 | 56.25 | 63.16 | |
| Former | 27.75 | 29.06 | 25 | |
| Current | 13.77 | 14.69 | 11.84 | |
| Alcohol use (g/day), mean (SD) | 14.9 (20.5) | 16.8 (22.6) | 10.9 (14.5) | <.001 |
| Diseases | ||||
| Coronary heart disease, % | 8.3 | 6.3 | 12.6 | 0.001 |
| Peripheral arterial disease, % | 6.1 | 4.1 | 10.5 | 0.001 |
| Type 2 Diabetes, % | 12.6 | 6.1 | 26.3 | <.001 |
| Lung disease, % | 9.3 | 8.6 | 10.9 | 0.26 |
| Osteoarthritis, % | 11.2 | 10.9 | 11.8 | 0.68 |
| Medications | ||||
| Antihypertensive drug use, % | 41.3 | 34.4 | 55.9 | <.001 |
| Lipid-lowering drug use, % | 4.8 | 2.2 | 10.2 | <.001 |
| Anti-diabetic drug use, % | 7.1 | 3.8 | 14.1 | <.001 |
| Metabolic syndrome components | ||||
| Blood pressure ≥130/85 mmHg†, % | 84.2 | 78.3 | 96.7 | <.001 |
| Waist circumference M: ≥102 cm / F: ≥ 88 cm, % |
40.5 | 23.0 | 77.3 | <.001 |
| Triglycerides ≥ 150 mg/dL‡, % | 28.4 | 11.6 | 63.8 | <.001 |
| HDL Cholesterol M: <40 mg/dL / F: <50 mg/dL, % |
21.7 | 5.9 | 54.9 | <.001 |
| Blood glucose ≥ 100 mg/dL§, % | 27.0 | 13.4 | 55.6 | <.001 |
| Adipocytokines | ||||
| CRP (mg/L), median (IQR) | 2.6 (1.3–5.4) | 2.3 (1.2–4.8) | 3.3 (1.8–6.3) | <.001 |
| IL-6 (pg/mL), median (IQR) | 1.4 (0.8–2.1) | 1.3 (0.8–2.0) | 1.6 (0.9–2.5) | <.001 |
| IL-1ra (pg/mL), median (IQR) | 133.0 (96.4–184.5) |
120.9 (91.0–164.6) |
166.8 (120.2–229.8 |
<.001 |
| IL-18 (pg/mL), median (IQR) | 380.7 (300.9–474.5) |
375.5 (287.2–467.8) |
398.0 (315.2–507.2) |
0.007 |
| TNF-α R1 (pg/mL), median (IQR) | 1330.8 (1089.0–1694.4) |
1298.9 (1060.0–1595.3) |
1428.9 (1137.6–1921.0) |
<.001 |
| Adiponectin (ng/mL), median (IQR) |
11.5 (7.0–17.3) |
12.7 (8.0–19.0) |
8.9 (5.6–14.3) |
<.001 |
| Leptin (ng/mL), median (IQR) | 8.8 (4.1–16.6) |
6.8 (3.4–14.3) |
12.4 (7.7–24.0) |
<.001 |
| Resistin (ng/mL), median (IQR) | 3.5 (2.7–4.7) | 3.5 (2.7–4.6) | 3.7 (2.7–5.1) | 0.04 |
Notes: Values are shown in mean (SD) and medians (IQR) for continuous variables and N (%) for categorical variables. MetS = metabolic syndrome; SD = standard deviation; HDL = high-density lipoprotein cholesterol; IQR = inter-quartile range; CRP = C-reactive protein; IL-6 = interleukin 6; IL-1ra = interleukin 1 receptor antagonist; IL-18 = interleukin 18, TNF-α R1 = tumor necrosis factor-α receptor 1.
Difference between No MetS and MetS
or use of anti-hypertensive medication
or use of lipid-lowering medication
or use of anti-diabetic medication.
The correlation coefficients between adipocytokines and metabolic determinants are shown in Table 2. All adipocytokines were associated with waist circumference (p < 0.05) and all except resistin were associated with glucose and triglycerides (p < 0.05). Furthermore, all adipocytokines except leptin were consistently associated with HDL serum levels. Serum levels of CRP, IL-6, IL-1ra, TNF-α R1 and resistin were positively associated with systolic blood pressure (p < 0.05).
Table 2.
Spearman correlation coefficients between adipocytokines and metabolic determinants
| Waist circumference (cm) |
Blood glucose (mg/dL) |
Triglycerides (mg/dL) |
HDL Cholesterol (mg/dL) |
Systolic blood pressure (mmHg) |
|
|---|---|---|---|---|---|
| CRP (mg/L) | 0.29*** | 0.09** | 0.12*** | −0.20*** | 0.08* |
| IL-6 (pg/mL) | 0.12*** | 0.07* | 0.11*** | −0.19*** | 0.15*** |
| IL-1ra (pg/mL) | 0.21*** | 0.10*** | 0.27*** | −0.33*** | 0.07* |
| IL-18 (pg/mL) | 0.15*** | 0.14*** | 0.13*** | −0.26*** | 0.05 |
| TNF-α R1 (pg/mL) | 0.12*** | 0.07* | 0.09** | −0.25*** | 0.11*** |
| Adiponectin (ng/mL) | −0.25*** | −0.19*** | −0.29*** | 0.42*** | 0.04 |
| Leptin (ng/mL) | 0.34*** | 0.13*** | 0.18*** | −0.02 | 0.06 |
| Resistin (ng/mL) | 0.06* | 0.01 | 0.03 | −0.16*** | 0.11*** |
p ≤ 0.05
p ≤ 0.01
p ≤ 0.001.
HDL = high-density lipoprotein cholesterol; CRP = C-reactive protein; IL-6 = interleukin 6; IL-1ra = interleukin 1 receptor antagonist; IL-18 = interleukin 18; TNF-α R1 = tumor necrosis factor- α receptor 1.
The associations of obesity, adipocytokines and the MetS are examined in Table 3. In an age and sex adjusted model (Model 1), both overweight (odds ratio [OR] = 2.50; 95% confidence interval [CI] 1.67–3.74) and obesity (OR = 8.73; 5.64–13.50) were associated with increased likelihood of having the MetS. After additionally adjusting for cytokines and adipokines (Model 3), the odds ratio for the MetS decreased 19% to 2.03 (95% CI 1.21–3.39) in overweight and 36% to 5.62 (95% CI 3.12–10.15) in obese participants. Moreover, IL-1ra and adiponectin remained significantly associated with the MetS after additionally adjusting for education and lifestyle factors (Model 4).
Table 3.
Odds ratios for the metabolic syndrome associated with body mass index and the mediating role of adipocytokines
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| BMI | ||||||||
| 18.5–24.9 kg/m2 (ref) (n=268) | 1 | 1 | 1 | |||||
| 25–29.9 kg/m2 (n=440) | 2.50 | 1.67–3.74 | 2.03 | 1.21–3.39 | 2.06 | 1.22–3.47 | ||
| ≥ 30 kg/m2 (n=236) | 8.73 | 5.64–13.50 | 5.62 | 3.12–10.15 | 5.27 | 2.89–9.59 | ||
| Cytokines * | ||||||||
| Log CRP (mg/L) | 1.17 | 0.95–1.45 | 1.12 | 0.90–1.39 | 1.08 | 0.87–1.35 | ||
| Log IL-6 (pg/mL) | 1.00 | 0.81–1.23 | 1.01 | 0.82–1.25 | 0.97 | 0.78–1.21 | ||
| Log IL-1ra (pg/mL) | 1.38 | 1.10–1.73 | 1.34 | 1.07–1.67 | 1.52 | 1.19–1.95 | ||
| Log IL-18 (pg/mL) | 1.15 | 0.95–1.39 | 1.17 | 0.96–1.42 | 1.17 | 0.95–1.43 | ||
| Log TNF-α R1 (pg/mL) | 1.27 | 1.02–1.57 | 1.27 | 1.01–1.58 | 1.22 | 0.97–1.54 | ||
| Adipokines * | ||||||||
| Log Adiponectin (ng/mL) | 0.56 | 0.46–0.67 | 0.54 | 0.44–0.66 | 0.54 | 0.44–0.66 | ||
| Log Leptin (ng/mL) | 1.85 | 1.47–2.34 | 1.29 | 0.99–1.68 | 1.26 | 0.96–1.65 | ||
| Log Resistin (ng/mL) | 1.01 | 0.83–1.22 | 1.03 | 0.84–1.26 | 1.01 | 0.82–1.24 | ||
Adipocytokines are presented as standardized values and OR indicates odds per standard deviation (SD) increment in adipocytokine. Model 1 included BMI, age and sex. Model 2 included adipocytokines, age and sex. Model 3 included BMI, adipocytokines, age and sex. Model 4 included BMI, adipocytokines, age, sex, education, physical activity, smoking and alcohol consumption.
OR = odds ratio; CI = confidence interval; BMI = body mass index; CRP = C-reactive protein (standard deviation SD=1.05 mg/L); IL-6 = interleukin 6 (SD=0.84 pg/mL); IL-1ra = interleukin 1 receptor antagonist (SD=0.57 pg/mL); IL-18 = interleukin 18 (SD=0.35 pg/mL), TNF-α R1 = tumor necrosis factor- α receptor 1 (SD=0.35 pg/mL). Adiponectin SD=0.73 ng/mL; Leptin SD=1.04 ng/mL; Resistin SD=0.45 ng/mL.
Parallel analyses carried out with waist circumference, as a measure of abdominal obesity, showed results that were very similar to BMI (Table 4). In addition to high waist circumference, IL-1ra and adiponectin remained associated with the MetS in models including age, sex, cytokines and adipokines. Persons with large waist circumference had elevated odds of the MetS (OR = 3.25; 95% CI 1.64–6.44) in the model including age, sex and adipocytokines, which was a 25% reduction from the age and sex adjusted model.
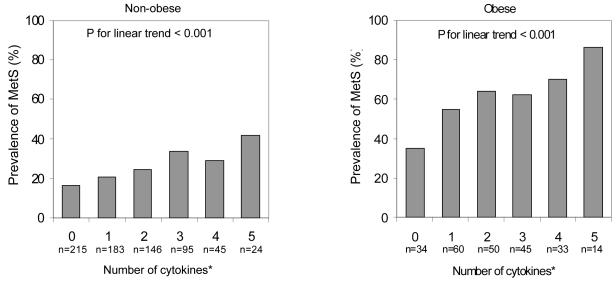
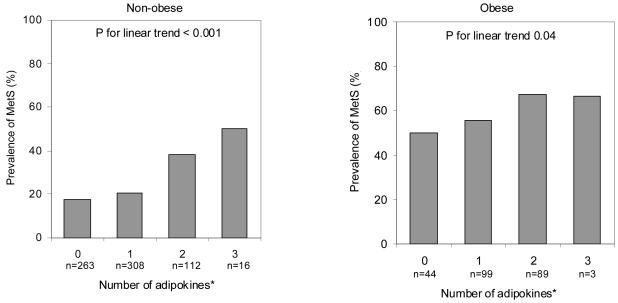
Next, the role of obesity status in the association between adipocytokines and the MetS was examined. As shown in Figure 1 and Figure 2, the prevalence of the MetS increased linearly with an increasing number of dysregulated cytokines and adipokines among both obese and non-obese persons. The MetS prevalence was higher among non-obese persons with five dysregulated cytokines (41.6%) compared to obese persons with no dysregulated cytokines (35.3%). In terms of dysregulated adipokines the prevalence of the MetS ranged from 17.6% to 50.3% in non-obese and from 50.0% to 66.7% in obese persons.
Figure 1.
Age-adjusted prevalence of the metabolic syndrome (MetS) by number of dysregulated cytokines (CRP, IL-6, IL-1ra, IL-18, TNF-α R1) in the highest tertile among non-obese and obese persons*.
Figure 2.
Age-adjusted prevalence of the metabolic syndrome (MetS) by number of dysregulated adipokines in the highest (leptin and resistin) and lowest for adiponectin tertile among non-obese and obese persons*.
In Table 5, the likelihood of the MetS among non-obese and obese persons is examined. In age and sex adjusted models, odds of the MetS increased linearly with an increasing number of dysregulated cytokines and adipokines. In non-obese persons, those with 2, 3 and 4–5 dysregulated cytokines had increased likelihood of the MetS (OR = 2.00, 95% CI 1.17–3.44; OR = 3.28, 95% CI 1.83–5.87 and OR = 3.76, 95% CI 1.91–7.42) compared to persons with no dysregulated cytokines. Similarly in obese persons the odds for the MetS were increased among those with 2, 3 and 4–5 dysregulated cytokines (OR = 3.57, 95% CI 1.42–9.00; OR = 3.36, 95% CI 1.31–8.61 and OR = 5.97, 95% CI 2.24–15.94). The cumulative number of dysregulated adipokines was associated with increased likelihood of the MetS only in non-obese persons (OR = 3.13, 95% CI 1.93–5.07). After adjusting for co-variates and insulin resistance measured with HOMA-IR, insulin resistance remained significant in each fully adjusted model (Table 5, Model 2) indicating a close association with the MetS. In comparing likelihood of the MetS between non-obese and obese persons, no interaction between obesity status and number of cytokines (p = 0.81) or obesity status and number of adipokines (p = 0.35) on the MetS was found.
Table 5.
Odds ratios for the metabolic syndrome according to the number of dysregulated adipocytokines among non-obese and obese persons
| Non-obese | Obese | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Number of cytokines * | ||||||||
| 0 (ref) | 1 | 1 | 1 | 1 | ||||
| 1 | 1.52 | 0.90–2.55 | 1.17 | 0.66–2.05 | 2.39 | 0.99–5.76 | 3.15 | 1.18–8.44 |
| 2 | 2.00 | 1.17–3.44 | 1.66 | 0.93–2.96 | 3.57 | 1.42–9.00 | 4.39 | 1.55–12.43 |
| 3 | 3.28 | 1.83–5.87 | 2.73 | 1.46–5.09 | 3.36 | 1.31–8.61 | 3.73 | 1.26–11.02 |
| 4–5 | 3.76 | 1.91–7.42 | 2.47 | 1.20–5.08 | 5.97 | 2.24–15.94 | 6.97 | 2.32–20.93 |
| Log HOMA-IR | 3.26 | 2.24–4.75 | 4.37 | 2.35–8.15 | ||||
| p for trend † | <.0001 | 0.001 | 0.001 | 0.002 | ||||
| Number of adipokines ‡ | ||||||||
| 0 (ref) | 1 | 1 | 1 | 1 | ||||
| 1 | 1.28 | 0.83–1.95 | 1.25 | 0.79–1.98 | 1.25 | 0.61–2.54 | 0.83 | 0.36–1.88 |
| 2–3 | 3.13 | 1.93–5.07 | 2.89 | 1.71–4.87 | 2.03 | 0.97–4.26 | 1.32 | 0.58–3.04 |
| Log HOMA-IR | 3.17 | 2.17–4.62 | 4.39 | 2.41–8.01 | ||||
| p for trend † | <.0001 | 0.0002 | 0.04 | 0.35 | ||||
Number of cytokines in the highest tertile (CRP, IL-6, IL-1ra, IL-18, TNF-α R1).
Trend test is examining the linearity of the ORs for number of cytokines and adipokines.
Number of adipokines in the highest tertile (leptin, resistin) and lowest tertile (adiponectin). Model 1 included adipocytokines, age and sex. Model 2 included adipocytokines,HOMA-IR, age, sex, education, physical activity, smoking and alcohol consumption. OR = odds ratio; CI = confidence interval; HOMA-IR = homeostasis model assessment indicating insulin resistance.
In analysis of individual adipocytokines after adjusting for insulin resistance and for co-variates, increased levels of IL-1ra and TNF-α R1 as well as decreased level of adiponectin were associated with higher likelihood of the MetS in both non-obese and obese persons (p < 0.05) (Table 6). In addition, among non-obese persons, increased levels of CRP, IL-6 and leptin were independently associated with the MetS (p < 0.05).
Table 6.
The likelihood of metabolic syndrome according to individual cytokines and adipokines among non-obese and obese
| Non- obese |
Obese | Non-obese | Obese | ||||
|---|---|---|---|---|---|---|---|
| Median | Median | p value* |
OR† | 95% CI | OR† | 95% CI | |
| Cytokines | |||||||
| Log CRP (mg/L) | 2.23 | 3.75 | <.0001 | 1.27 | 1.05–1.53 | 1.22 | 0.89–1.67 |
| Log IL-6 (pg/mL) | 1.37 | 1.59 | 0.02 | 1.30 | 1.01–1.67 | 1.39 | 0.92–2.12 |
| Log IL-1RA (pg/mL) | 125.74 | 165.20 | <.0001 | 2.33 | 1.57–3.45 | 2.55 | 1.45–4.50 |
| Log IL-18 (pg/mL) | 378.10 | 390.32 | 0.21 | 1.50 | 0.82–2.73 | 2.42 | 0.89–6.52 |
| Log TNF-a R1 (pg/mL) | 1303.80 | 1411.90 | 0.001 | 3.04 | 1.58–5.88 | 2.77 | 1.05–7.36 |
| Adipokines | |||||||
| Log Adiponectin (ng/mL) | 11.74 | 10.38 | 0.03 | 0.37 | 0.27–0.51 | 0.55 | 0.32–0.93 |
| Log Leptin (ng/mL) | 7.014 | 16.80 | <.0001 | 1.47 | 1.14–1.89 | 1.18 | 0.76–1.84 |
| Log Resistin (ng/mL) | 3.50 | 3.70 | 0.06 | 1.50 | 0.95–2.36 | 1.51 | 0.72–3.17 |
Median differences examined with Kruskal-Wallis.
Models are adjusted for age, sex, education, physical activity, smoking, alcohol consumption and HOM-IR
Subsequent analyses were performed to understand whether association between dysregulated adipocytokines and the MetS were different in normal weight and overweight groups. The MetS was present in 14.6% of normal weight persons and 28.4% of overweight persons. In logistic regression models including HOMA-IR and co-variates, an increasing number of dysregulated cytokines was linearly associated with increasing likelihood of the MetS among both normal weight (p for trend 0.003) and overweight persons (p for trend 0.04). Similarly, an increasing number of adipokines was linearly associated with the MetS in both normal weight (p for trend 0.02) and overweight persons (p for trend 0.02).
Finally, to control for the confounding effect of diabetes on the studied associations, similar analyses were conducted among non-diabetics (n = 825). Results were comparable with the results based on the original study population.
DISCUSSION
In this population-based study, we demonstrated that adipocytokines partially account for the association between obesity and the MetS. Interestingly, we also found that elevated levels of inflammatory cytokines increased the likelihood of having the MetS among both obese and non-obese persons independent of insulin resistance estimated by the HOMA-IR. Finally, of the large number of adipocytokines included, high levels of IL-1ra and TNF-α R1 and low levels of adiponectin were most strongly and independently associated with the MetS.
The results of this study also confirm current views about the association of obesity, inflammatory status and the MetS. Adipose tissue-derived inflammatory markers, such as CRP, IL-6, IL-1ra and TNF-α, have been associated either with individual metabolic alterations or the MetS 7, 13, 14. In addition, leptin and adiponectin, both derived from adipose tissue, are associated with the MetS 7, 21-23. The present study extended this line of research by examining several adipocytokines simultaneously and by examining the association between adipocytokines and the MetS independent of overall and central obesity. In this study we used combined measures of multiple cytokines and adipokines to indicate inflammatory burden. Because the MetS results from alterations in multiple systems, using a combination of inflammatory markers instead of a single marker increases the chance of capturing the severity of inflammation, as well as overall and interactive effects of inflammatory markers on the metabolic alterations.
Our results showing an association between IL-1ra, TNF-α R1, adiponectin and the MetS independent of obesity status are consistent with previous literature. Increased levels of circulating IL-1ra and TNF-α R1 are associated with the MetS and with insulin resistance independent of obesity 7, 23-25. Soluble receptors for cytokines, such as IL-1ra and TNF-α R1, have a longer half-life than directly measured IL-6 and TNF-α, which may allow them to prolong the biological effects of the cytokines 26. In addition, they are considered sensitive markers of cytokine response because of the better measurement accuracy 16, 17. Furthermore, a strong association between low circulating levels of adiponectin and the MetS has been reported 14, 27 and the association may be due to the diminished insulin-sensitizing actions of adiponectin and its participation in glucose and lipid metabolism 28. As seen in this study, waist circumference and insulin resistance estimated by the HOMA-IR are closely correlated with the MetS as well as with several adipocytokines. The inflammatory factors secreted from adipose tissue contribute considerably to obesity’s metabolic complications as well as to development of insulin resistance. In fact, it has been suggested that inflammation should be added as one of the criterion for the MetS 29.
The unique contribution of the current study is the attempt to distinguish between “simple” and “complicated” obesity. As the present study shows, not all obese individuals have metabolic alterations, and metabolic alterations can also exist independent of obesity. Based on our findings, we suggest that whether or not obese individuals develop the MetS depends on the degree of concurrent inflammatory state. Furthermore, because similar associations between cytokines and the MetS were observed in normal weight and overweight participants, the effect of cytokines seems to be independent of overall obesity status. Only one previous epidemiological study has examined the association of several adipocytokines and MetS with different levels of adiposity 7. In their study, You et al. 7 reported that circulating adipocytokines, such as CRP, IL-6 and TNF-α were significantly higher among persons with the MetS compared to those without the MetS across body fat percentage tertiles. The differences in CRP and IL-6 levels between persons with and without MetS were more pronounced in the highest body fat percentage tertile. Our results also confirm the findings of a few earlier smaller-scale studies that report higher levels CRP 30 and TNF-α R1 24 in obese women with the MetS compared to obese persons without MetS.
No independent association between cumulative number of dysregulated adipokines and the MetS was found in the obese population, although this association was found among non-obese persons. This finding is difficult to explain based on current knowledge. Kantartzis and co-workers have reported that associations between adiponectin and insulin resistance, subclinical inflammation and dyslipidemia are stronger among obese non-diabetic middle-aged persons than lean persons 31, 32. On the other hand, You and co-workers have reported that adiponectin and leptin are associated with the MetS across body fat percentage tertiles 7. In terms of the individual adipokines in the present study, adiponectin was significantly associated with the MetS in both non-obese and obese persons, whereas leptin was significantly associated with the MetS only among non-obese persons. Resistin was not associated with the MetS either obese or non-obese persons. Overall, in humans the relationship between resistin and metabolic alterations is disputed 15, 23, 33. One explanation for our findings of a non-significant association between the number of dysregulated adipokines and the MetS may lay in the existence of a ceiling effect for the adiponectin and leptin levels in obese persons. Because both of these markers are strongly associated with the amount of fat mass, a higher number of obese persons were placed in the dysregulated category (lowest tertile for adiponectin and highest tertile for leptin), making it more difficult to observe a dose-effect response in the outcome variable.
It is important to identify the causes of variation in inflammatory profiles across obesity status. What protects some obese persons from developing an increased inflammatory profile? What risk factors predispose some normal weight persons to high levels of inflammation and increased likelihood of metabolic alterations? One plausible hypothesis is that fat distribution between subcutaneous and visceral adipose depots may cause different inflammatory responses and susceptibility to metabolic alterations among both obese and non-obese persons 34. Similarly, different aspects of fatty liver vary in their association with inflammation and metabolic consequences. For example, preferential hepatic fat storage in the form of triglycerides, but not long chain Acyl-coenzyme A, appears to protect from subclinical inflammation and the metabolic syndrome in obesity 35. Small studies have shown that obese persons without MetS have lower visceral fat content 24, 30 as well as less fat in the skeletal muscle and liver 2 compared to obese persons with MetS. This makes sense physiologically, because fat accumulation in the insulin-sensitive tissues of liver or muscle is associated with insulin resistance and other features of MetS independent of overall adiposity 36. Unfortunately, this hypothesis could not be tested in our study because we did not have dual-energy x-ray absorptiometry (DXA) or computed tomography available to gain additional insight about the total body fat quantity and distribution.
In addition to the potential differences in body composition, differences in quantity, intensity and type of physical activity may explain differences in inflammatory profiles among obese persons. It has been widely shown in both observational and intervention studies that long term physical activity leads to lower circulating levels of inflammatory markers, such as CRP, IL-6 and TNF-α, and to higher levels of adiponectin, especially when total body fat mass is also decreased 37-39. Exercise training also improves insulin sensitivity and HDL levels as well as decreases triglyceride level and blood pressure 40-42. In studies comparing non-obese persons with and without insulin resistance, lower energy expenditure and lower peak oxygen uptake have been reported among subjects with insulin resistance 43. In the current study, we controlled for physical activity, but it did not contribute much to the excess likelihood of the MetS. However, adjusting for cardiorespiratory fitness might have been more useful. The inverse association between cardiorespiratory fitness and health outcomes is generally stronger and more consistent across studies than the relationship between physical activity and health outcomes 44.
More prospective and genetic studies are needed to examine factors, such as body composition and physical activity, which can explain the underlying differences in inflammatory response among obese and non-obese people. Moreover, from a public health perspective, an important area for future studies is to investigate the health risks related to different combinations of obesity and MetS. So far there is some evidence that the risk of cardiovascular events and functional decline is lower among obese persons without metabolic alterations compared to obese persons with several metabolic alterations, as well as among non-obese persons without metabolic alterations compared to non-obese persons with metabolic alterations 45 46. However, it is not known whether obese persons without the MetS have lower risk of mortality or what the health risks may be among non-obese persons with the MetS.
Limitations in the present study warrant discussion. First, the cross-sectional design does not allow us to determine whether increased inflammation is a cause or consequence of metabolic alterations and/or obesity. However, the current understanding is that excess adiposity leads to increases in circulating levels of inflammatory cytokines and decreases in level of adiponectin 47, which further enhance the risk of developing metabolic alterations 48. Second, due to lack of objective body composition measures, we were not able to examine the role of total body fat and visceral fat on the association between adipocytokines and the MetS. Third, we evaluated fasting insulin and HOMA-IR as surrogates for insulin resistance. However, the HOMA-IR index has been validated as an indicator of insulin resistance in several clinical and epidemiological studies 11, 12, 49. Fourth, the ATP-III criteria were used to define the MetS, but there is ongoing dispute about the definition and concept of the MetS 50. The use of the MetS as an outcome can be justified because it is related to increased risk of type 2 diabetes and cardiovascular diseases 51. Finally, because the InCHIANTI Study consists mainly of older Caucasians, the findings may have limited generalizability to other ethnic and age groups.
In conclusion, altered levels of adipocytokines are associated with the MetS both among obese and non-obese individuals independent of insulin resistance estimated by the HOMA-IR. Further studies are needed to understand the role of body composition in promoting an increased inflammatory response leading to metabolic alterations.
Acknowledgements
The InCHIANTI study baseline (1998–2000) was supported as a “targeted project” (ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336) and by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland. This work was also supported by grants from the Finnish Academy (no. 125494 SS). None of the sponsoring institutions interfered with the collection, analysis, presentation, or interpretation of the data reported here.
Footnotes
Competing interests/financial disclosure
The authors declare no conflict of interest.
References
- 1.World Health Organization . Report of a WHO Consultation. Geneva: 2000. Obesity: Preventing and managing the global epidemic. WHO Technical Report Series 894. [PubMed] [Google Scholar]
- 2.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Haring HU. Identification and characterization of metabolically benign obesity in humans. Archives of Internal Medicine. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 3.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Archives of Internal Medicine. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 4.Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET. Metabolic and body composition factors in subgroups of obesity: what do we know? Journal of Clinical Endocrinology & Metabolism. 2004;89:2569–2575. doi: 10.1210/jc.2004-0165. [DOI] [PubMed] [Google Scholar]
- 5.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. European Cytokine Network. 2006;17:4–12. [PubMed] [Google Scholar]
- 6.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 7.You T, Nicklas BJ, Ding J, Penninx BW, Goodpaster BH, Bauer DC, Tylavsky FA, Harris TB, Kritchevsky SB. The metabolic syndrome is associated with circulating adipokines in older adults across a wide range of adiposity. The journals of gerontology. Series A, Biological sciences and medical sciences. 2008;63:414–419. doi: 10.1093/gerona/63.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macci C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. Journal of American Geriatrics Society. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 9.WHO . Report of a WHO Consultation. Geneva, Switzerland: 2000. Obesity: Preventing and managing the global epidemic. [PubMed] [Google Scholar]
- 10.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr., Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 13.Hung J, McQuillan BM, Chapman CM, Thompson PL, Beilby JP. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:1268–1273. doi: 10.1161/01.ATV.0000163843.70369.12. [DOI] [PubMed] [Google Scholar]
- 14.Hung J, McQuillan BM, Thompson PL, Beilby JP. Circulating adiponectin levels associate with inflammatory markers, insulin resistance and metabolic syndrome independent of obesity. International journal of Obesity (2005) 2008;32:772–779. doi: 10.1038/sj.ijo.0803793. [DOI] [PubMed] [Google Scholar]
- 15.Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S64–73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabay C, Smith MF, Eidlen D, Arend WP. Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase protein. J Clin Invest. 1997;99:2930–2940. doi: 10.1172/JCI119488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dibbs Z, Thornby J, White BG, Mann DL. Natural variability of circulating levels of cytokines and cytokine receptors in patients with heart failure: implications for clinical trials. J Am Coll Cardiol. 1999;33:1935–1942. doi: 10.1016/s0735-1097(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 18.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. International Journal of Epidemiology. 1997;26(Suppl 1):S152–160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 19.Guralnik J, Fried L, Simonsick E, Kasper J, Lafferty M. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. National Institute on Aging; Bethesda, MD: 1995. NIH Publication No. 95-4009. [Google Scholar]
- 20.St-Onge MP, Janssen I, Heymsfield SB. Metabolic syndrome in normal-weight Americans: new definition of the metabolically obese, normal-weight individual. Diabetes Care. 2004;27:2222–2228. doi: 10.2337/diacare.27.9.2222. [DOI] [PubMed] [Google Scholar]
- 21.Zamboni M, Zoico E, Fantin F, Panourgia MP, Di Francesco V, Tosoni P, Solerte B, Vettor R, Bosello O. Relation between leptin and the metabolic syndrome in elderly women. J Gerontol A Biol Sci Med Sci. 2004;59:396–400. doi: 10.1093/gerona/59.4.m396. [DOI] [PubMed] [Google Scholar]
- 22.Maggio M, Lauretani F, Ceda GP, Bandinelli S, Basaria S, Ble A, Egan J, Paolisso G, Najjar S, Jeffrey Metter E, Valenti G, Guralnik JM, Ferrucci L. Association between hormones and metabolic syndrome in older Italian men. Journal of American Geriatrics Society. 2006;54:1832–1838. doi: 10.1111/j.1532-5415.2006.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hivert MF, Sullivan LM, Fox CS, Nathan DM, D’Agostino RB, Sr., Wilson PW, Meigs JB. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J Clin Endocrinol Metab. 2008;93:3165–3172. doi: 10.1210/jc.2008-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.You T, Ryan AS, Nicklas BJ. The metabolic syndrome in obese postmenopausal women: relationship to body composition, visceral fat, and inflammation. Journal of Clinical Endocrinology & Metabolism. 2004;89:5517–5522. doi: 10.1210/jc.2004-0480. [DOI] [PubMed] [Google Scholar]
- 25.Abbatecola AM, Ferrucci L, Grella R, Bandinelli S, Bonafe M, Barbieri M, Corsi AM, Lauretani F, Franceschi C, Paolisso G. Diverse effect of inflammatory markers on insulin resistance and insulin-resistance syndrome in the elderly. Journal of American Geriatrics Society. 2004;52:399–404. doi: 10.1111/j.1532-5415.2004.52112.x. [DOI] [PubMed] [Google Scholar]
- 26.Grell M. Tumor necrosis factor (TNF) receptors in cellular signaling of soluble and membrane-expressed TNF. J Inflamm. 1995;47:8–17. [PubMed] [Google Scholar]
- 27.Matsushita K, Yatsuya H, Tamakoshi K, Wada K, Otsuka R, Takefuji S, Sugiura K, Kondo T, Murohara T, Toyoshima H. Comparison of circulating adiponectin and proinflammatory markers regarding their association with metabolic syndrome in Japanese men. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:871–876. doi: 10.1161/01.ATV.0000208363.85388.8f. [DOI] [PubMed] [Google Scholar]
- 28.Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin--a key adipokine in the metabolic syndrome. Diabetes, obesity & metabolism. 2006;8:264–280. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109:2818–2825. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- 30.Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud’homme D, Rabasa-Lhoret R. The metabolically healthy but obese individual presents a favorable inflammation profile. Journal of Clinical Endocrinology & Metabolism. 2005;90:4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 31.Kantartzis K, Rittig K, Balletshofer B, Machann J, Schick F, Porubska K, Fritsche A, Haring HU, Stefan N. The relationships of plasma adiponectin with a favorable lipid profile, decreased inflammation, and less ectopic fat accumulation depend on adiposity. Clin Chem. 2006;52:1934–1942. doi: 10.1373/clinchem.2006.067397. [DOI] [PubMed] [Google Scholar]
- 32.Kantartzis K, Fritsche A, Tschritter O, Thamer C, Haap M, Schafer S, Stumvoll M, Haring HU, Stefan N. The association between plasma adiponectin and insulin sensitivity in humans depends on obesity. Obes Res. 2005;13:1683–1691. doi: 10.1038/oby.2005.206. [DOI] [PubMed] [Google Scholar]
- 33.Qasim AN, Metkus TS, Tadesse M, Lehrke M, Restine S, Wolfe ML, Hannenhalli S, Cappola T, Rader DJ, Reilly MP. Resistin gene variation is associated with systemic inflammation but not plasma adipokine levels, metabolic syndrome or coronary atherosclerosis in nondiabetic Caucasians. Clin Endocrinol (Oxf) 2009;70:698–705. doi: 10.1111/j.1365-2265.2008.03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, Nevitt M, Holvoet P, Newman AB. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Archives of Internal Medicine. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 35.Stefan N, Kantartzis K, Haring HU. Causes and metabolic consequences of Fatty liver. Endocr Rev. 2008;29:939–960. doi: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 36.Seppälä-Lindroos A, Vehkavaara S, Häkkinen AM, Goto T, Westerbacka J, Sovijärvi A, Halavaara J, Yki-Järvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. Journal of Clinical Endocrinology & Metabolism. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 37.Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, Rubin S, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 38.You T, Nicklas BJ. Effects of exercise on adipokines and the metabolic syndrome. Current diabetes reports. 2008;8:7–11. doi: 10.1007/s11892-008-0003-4. [DOI] [PubMed] [Google Scholar]
- 39.Kondo T, Kobayashi I, Murakami M. Effect of exercise on circulating adipokine levels in obese young women. Endocrine journal. 2006;53:189–195. doi: 10.1507/endocrj.53.189. [DOI] [PubMed] [Google Scholar]
- 40.Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, Nguyen-Duy TB, Lee S, Kilpatrick K, Hudson R. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obesity Research. 2004;12:789–798. doi: 10.1038/oby.2004.95. [DOI] [PubMed] [Google Scholar]
- 41.Thompson PD, Crouse SF, Goodpaster B, Kelley D, Moyna N, Pescatello L. The acute versus the chronic response to exercise. Medicine & Science in Sports & Exercise. 2001;33:S438–445. doi: 10.1097/00005768-200106001-00012. discussion S452–433. [DOI] [PubMed] [Google Scholar]
- 42.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. New England Journal of Medicine. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 43.Conus F, Allison DB, Rabasa-Lhoret R, St-Onge M, St-Pierre DH, Tremblay-Lebeau A, Poehlman ET. Metabolic and behavioral characteristics of metabolically obese but normal-weight women. J Clin Endocrinol Metab. 2004;89:5013–5020. doi: 10.1210/jc.2004-0265. [DOI] [PubMed] [Google Scholar]
- 44.Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Medicine & Science in Sports & Exercise. 2001;33:S379–399. doi: 10.1097/00005768-200106001-00007. discussion S419–320. [DOI] [PubMed] [Google Scholar]
- 45.St-Pierre AC, Cantin B, Mauriege P, Bergeron J, Dagenais GR, Despres JP, Lamarche B. Insulin resistance syndrome, body mass index and the risk of ischemic heart disease. Canadian Medical Association journal. 2005;172:1301–1305. doi: 10.1503/cmaj.1040834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stenholm S, Koster A, Alley D, Houston DK, Kanaya AM, Lee JS, Newman AB, Satterfield S, Simonsick E, Visser M, Harris T, Ferrucci L, Health, A.a.B.C.S. Joint association of obesity and metabolic syndrome with incident mobility limitation in older men and women - Results from the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2009 Oct 12; doi: 10.1093/gerona/glp150. for the. doi:10.1093/gerona/glp150. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 48.Reilly MP, Rader DJ. The metabolic syndrome: more than the sum of its parts? Circulation. 2003;108:1546–1551. doi: 10.1161/01.CIR.0000088846.10655.E0. [DOI] [PubMed] [Google Scholar]
- 49.Wallace TM, Matthews DR. The assessment of insulin resistance in man. Diabet Med. 2002;19:527–534. doi: 10.1046/j.1464-5491.2002.00745.x. [DOI] [PubMed] [Google Scholar]
- 50.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 51.Butler J, Rodondi N, Zhu Y, Figaro K, Fazio S, Vaughan DE, Satterfield S, Newman AB, Goodpaster B, Bauer DC, Holvoet P, Harris TB, de Rekeneire N, Rubin S, Ding J, Kritchevsky SB. Metabolic syndrome and the risk of cardiovascular disease in older adults. Journal of the American College of Cardiology. 2006;47:1595–1602. doi: 10.1016/j.jacc.2005.12.046. [DOI] [PubMed] [Google Scholar]