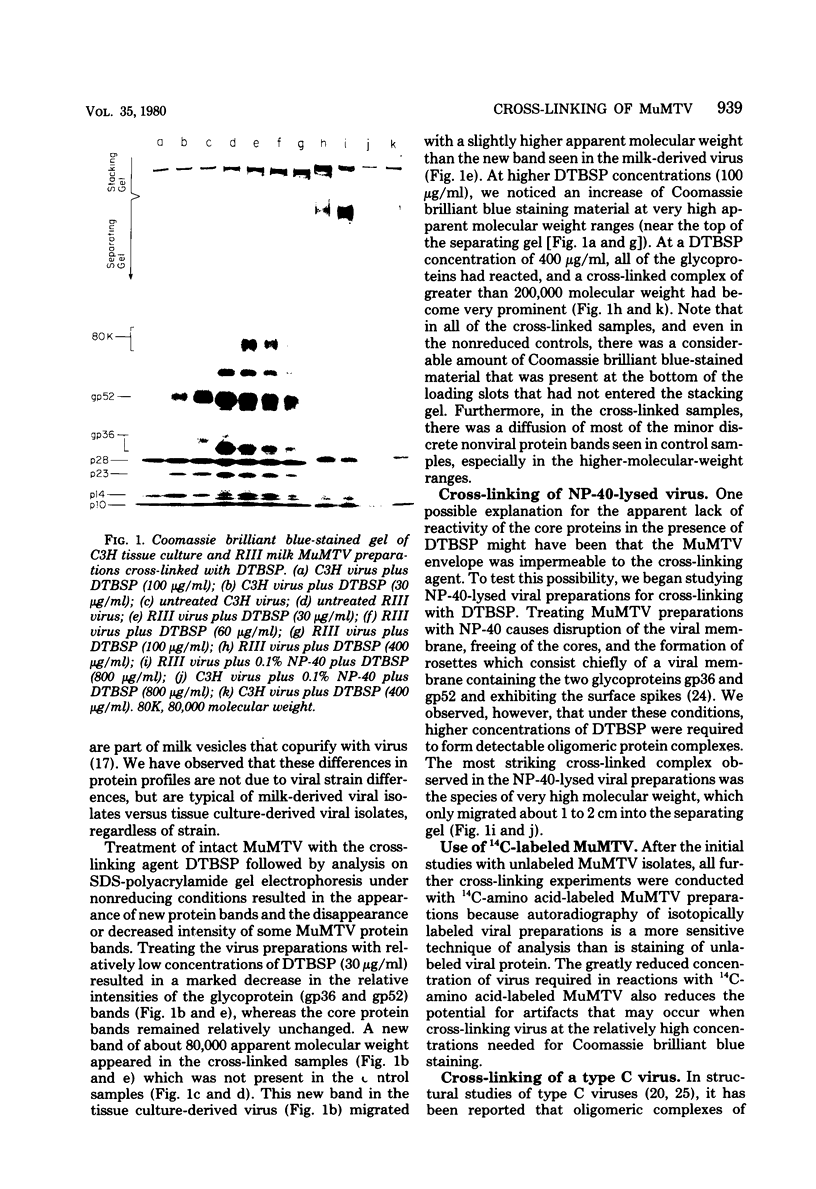
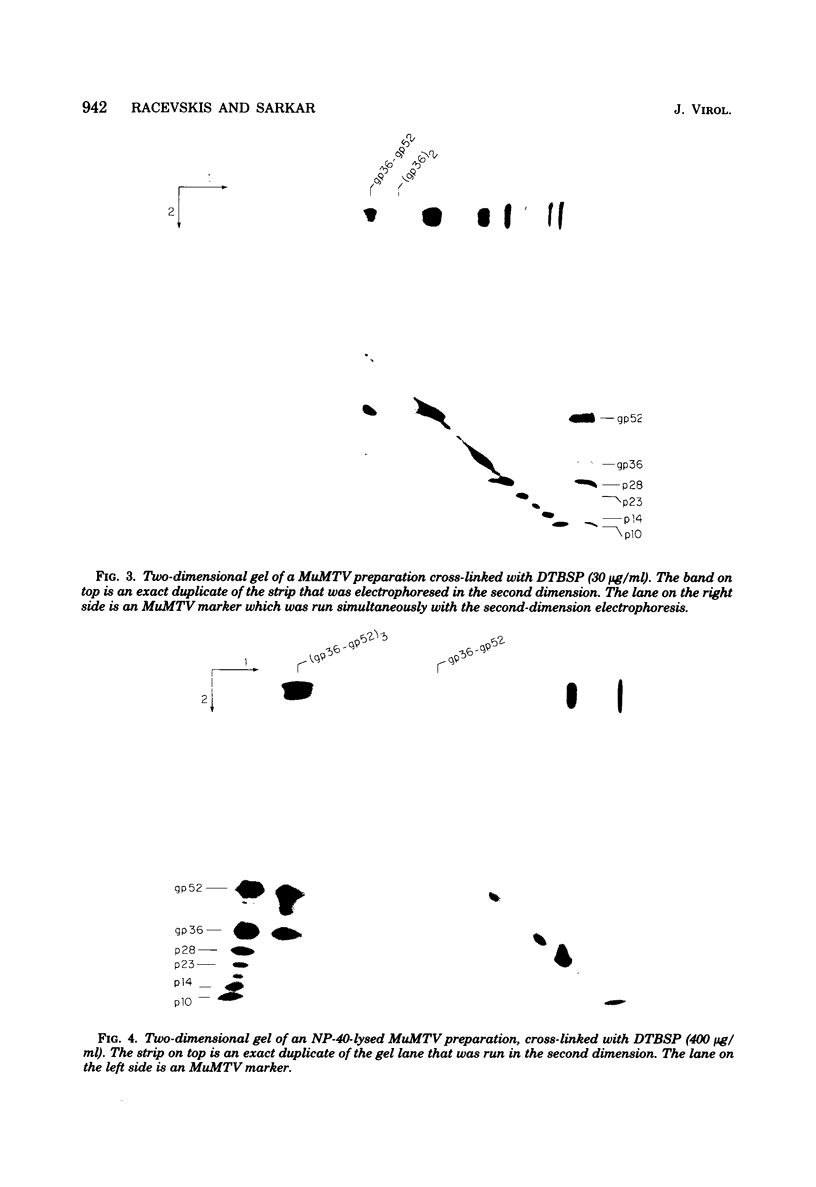
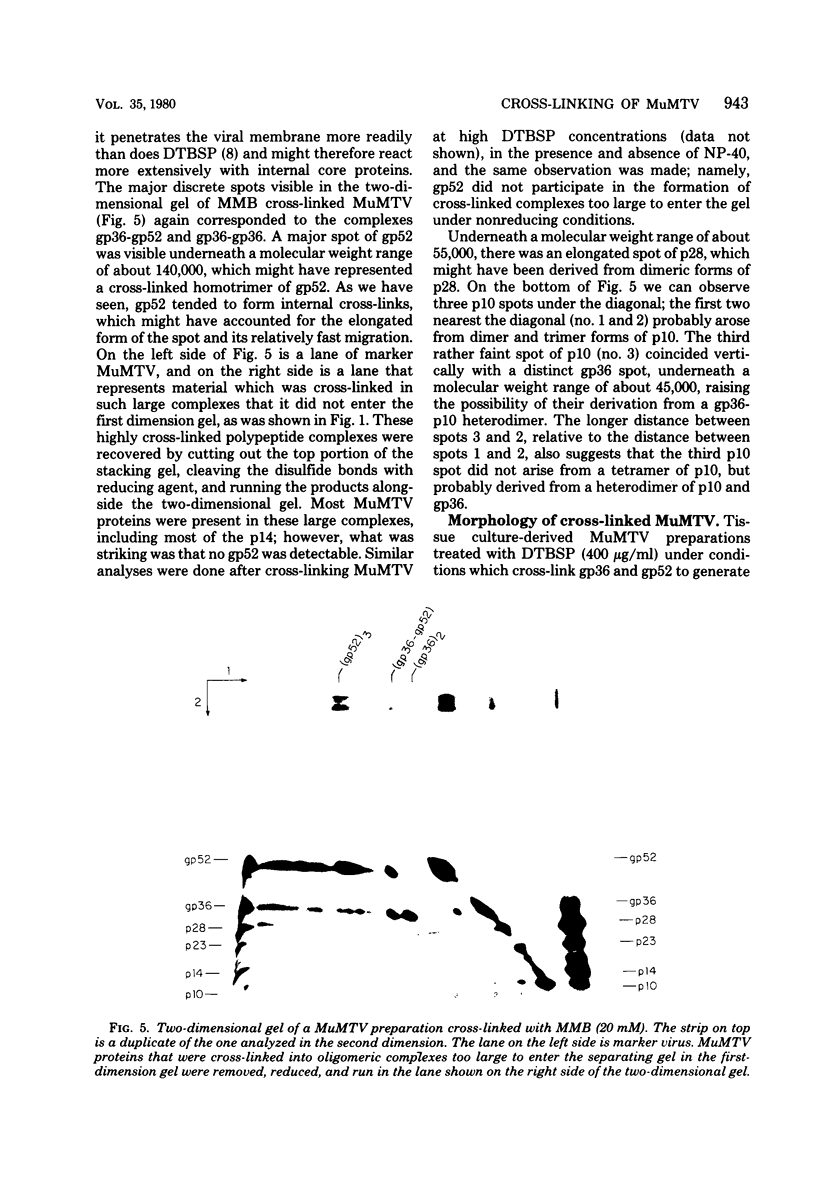
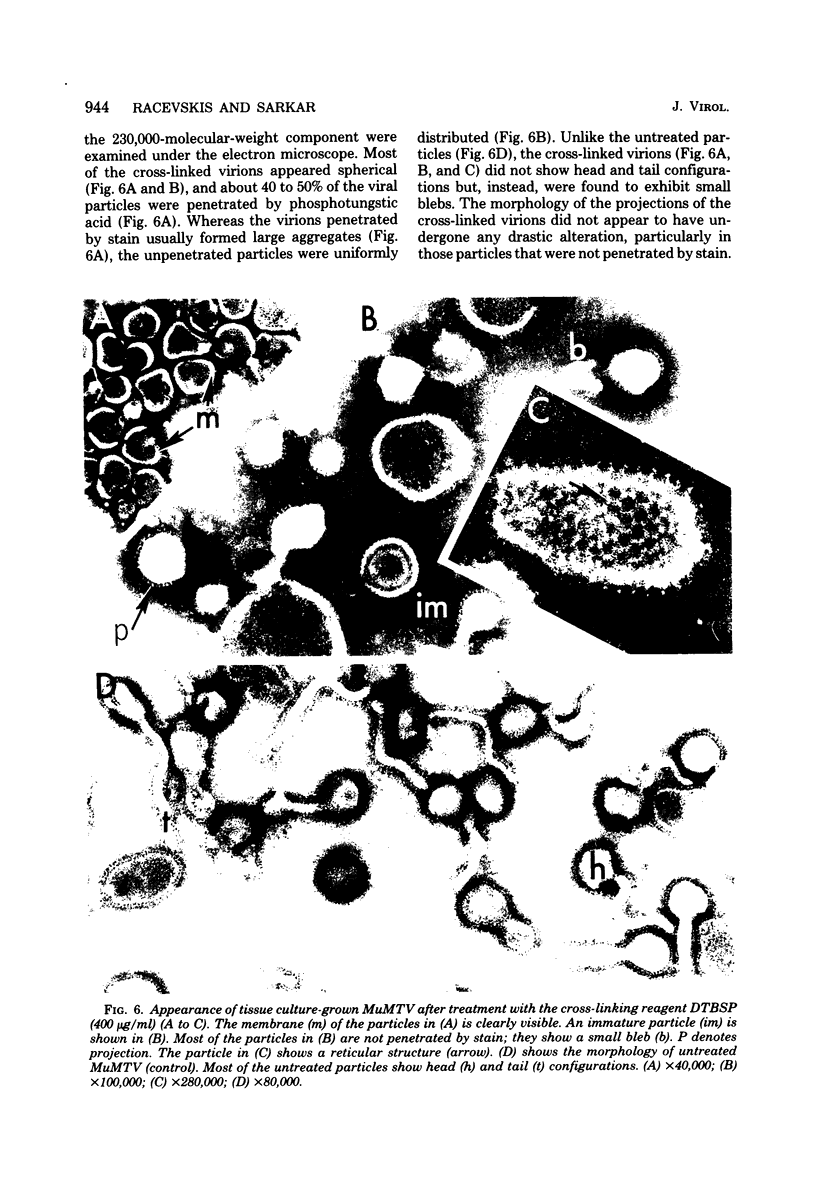
Abstract
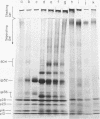
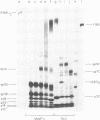
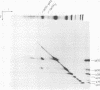
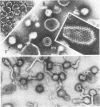
Murine mammary tumor virus protein interactions in the intact virion structure were studied with the use of the cleavable cross-linking reagents dithiobis(succinimidyl propionate) and methyl 4-mercaptobutyrimidate hydrochloride. Cross-linked oligomeric complexes of murine mammary tumor virus proteins were analyzed by two-dimensional gel electrophoresis. Among the complexes most consistently formed were a heterodimer of the two glycoproteins gp36 and gp52, the homodimer of gp36, and the homotrimer of gp52. A very prominent oligomer formed at higher concentrations of dithiobis(succinimidyl propionate) was a complex of about 230,000 molecular weight, made up of three molecules each of gp36 and gp52. A number of lines of evidence, including electron microscopic analysis, suggest that the 230,000-molecular-weight complex actually represents the murine mammary tumor virus spike structure. Of the murine mammary tumor virus core proteins, p14 forms homooligomers most readily. Upon cross-linking with methyl 4-mercaptobutyrimidate hydrochloride a small amount of what seems to be a heterodimer made up of the N-terminal gag protein p10 and the hydrophobic membrane glycoprotein gp36 can be observed.
Full text
PDF




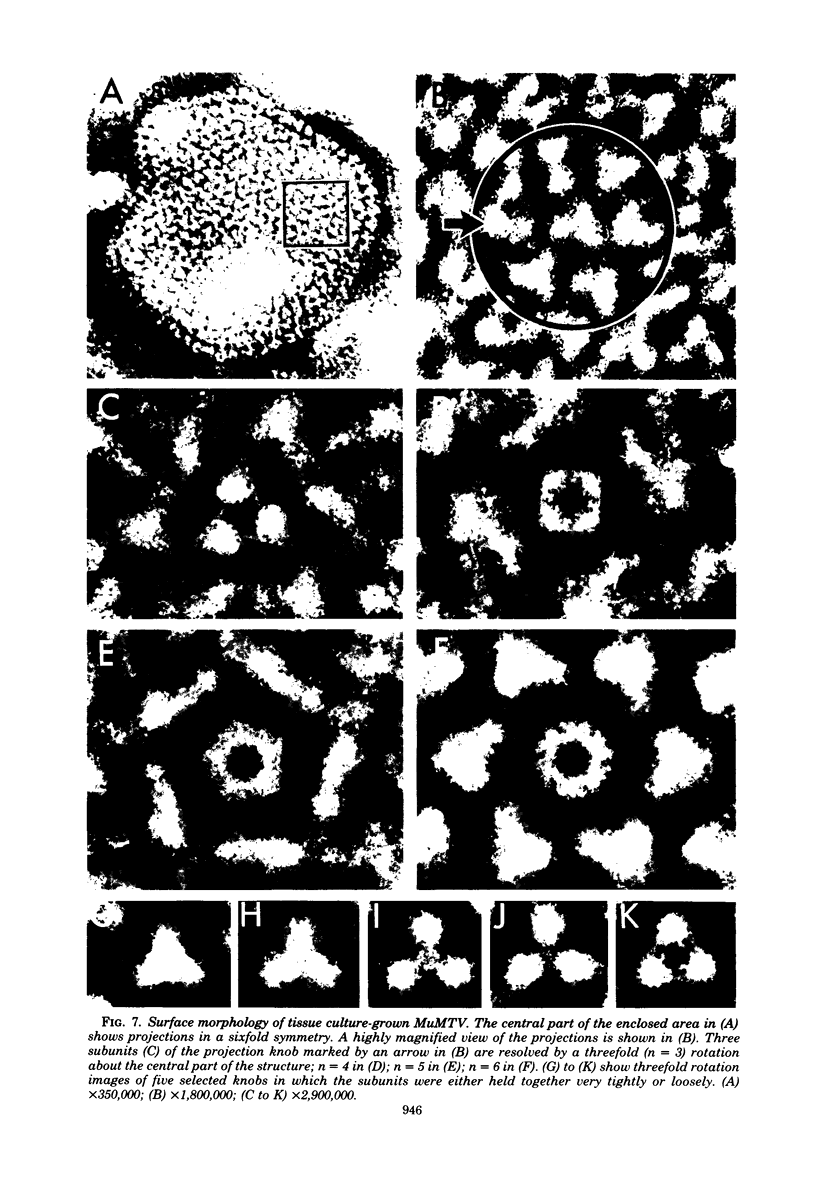


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur L. O., Long C. W., Smith G. H., Fine D. L. Immunological characterization of the low-molecular-weight DNA binding protein of mouse mammary tumor virus. Int J Cancer. 1978 Oct 15;22(4):433–440. doi: 10.1002/ijc.2910220411. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Montelaro R. C., Frank H., Schäfer W. Assembly of type C oncornaviruses: a model. Science. 1978 Jan 13;199(4325):183–186. doi: 10.1126/science.202022. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cardiff R. D., Puentes M. J., Young L. J., Smith G. H., Teramoto Y. A., Altrock B. W., Pratt T. S. Serological and biochemical characterization of the mouse mammary tumor virus with localization of p10. Virology. 1978 Mar;85(1):157–167. doi: 10.1016/0042-6822(78)90420-8. [DOI] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Composition, arrangement and cleavage of the mouse mammary tumor virus polyprotein precursor Pr77gag and p110gag. Cell. 1979 Aug;17(4):1003–1012. doi: 10.1016/0092-8674(79)90339-8. [DOI] [PubMed] [Google Scholar]
- Dion A. S., Pomenti A. A., Farwell D. C. Vicinal relationships between the major structural proteins of murine mammary tumor virus. Virology. 1979 Jul 15;96(1):249–257. doi: 10.1016/0042-6822(79)90188-0. [DOI] [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H. Cross-linking of the spike glycoproteins in Semliki Forest virus with dimethylsuberimidate. Virology. 1974 Dec;62(2):385–392. doi: 10.1016/0042-6822(74)90400-0. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Lerner R., Howard D., Teramoto Y. A., Schlom J. Strain-specific markers for the major structural proteins of highly oncogenic murine mammary tumor viruses by tryptic peptide analyses. J Virol. 1978 Sep;27(3):688–699. doi: 10.1128/jvi.27.3.688-699.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny J. W., Lambert J. M., Traut R. R. Cross-linking of ribosomes using 2-iminothiolane (methyl 4-mercaptobutyrimidate) and identification of cross-linked proteins by diagonal polyacrylamide/sodium dodecyl sulfate gel electrophoresis. Methods Enzymol. 1979;59:534–550. doi: 10.1016/0076-6879(79)59112-5. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Mosher D. F., Vaheri A. Dimeric character of fibronectin, a major cell surface-associated glycoprotein. Biochem Biophys Res Commun. 1977 Jan 24;74(2):699–706. doi: 10.1016/0006-291x(77)90359-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Marcus S. L., Smith S. W., Racevskis J., Sarkar N. H. The relative hydrophobicity of oncornaviral structural proteins. Virology. 1978 May 15;86(2):398–412. doi: 10.1016/0042-6822(78)90080-6. [DOI] [PubMed] [Google Scholar]
- Massey R. J., Schochetman G. Gene order of mouse mammary tumor virus precusor polyproteins and their interaction leading to the formation of a virus. Virology. 1979 Dec;99(2):358–371. doi: 10.1016/0042-6822(79)90015-1. [DOI] [PubMed] [Google Scholar]
- Moore D. H., Long C. A., Vaidya A. B., Sheffield J. B., Dion A. S., Lasfargues E. Y. Mammary tumor viruses. Adv Cancer Res. 1979;29:347–418. doi: 10.1016/s0065-230x(08)60850-7. [DOI] [PubMed] [Google Scholar]
- Mudd J. A., Swanson R. E. In situ cross-linking of vesicular stomatitis virus proteins with reversible agents. Virology. 1978 Jul 15;88(2):263–280. doi: 10.1016/0042-6822(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Identification of retrovirus matrix proteins by lipid-protein cross-linking. J Mol Biol. 1979 Jul 15;131(4):819–837. doi: 10.1016/0022-2836(79)90203-1. [DOI] [PubMed] [Google Scholar]
- Pinter A., Fleissner E. Structural studies of retroviruses: characterization of oligomeric complexes of murine and feline leukemia virus envelope and core components formed upon cross-linking. J Virol. 1979 Apr;30(1):157–165. doi: 10.1128/jvi.30.1.157-165.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racevskis J., Sarkar N. H. Synthesis and processing of precursor polypeptides to murine mammary tumor virus structural proteins. J Virol. 1978 Jan;25(1):374–383. doi: 10.1128/jvi.25.1.374-383.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. D., Vance D. E. Chemical cross-linking of proteins of Semliki Forest virus: virus particles and plasma membranes from BHK-21 cells treated with colchicine or dibucaine. J Virol. 1978 Oct;28(1):193–198. doi: 10.1128/jvi.28.1.193-198.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar N. H., Moore D. H. Surface structure of mouse mammary tumor virus. Virology. 1974 Sep;61(1):38–55. doi: 10.1016/0042-6822(74)90240-2. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Taraschi N. E., Pomenti A. A., Dion A. S. Polypeptides of the mouse mammary tumor virus. II. Identification of two major glycoproteins with the viral structure. Virology. 1976 Feb;69(2):677–690. doi: 10.1016/0042-6822(76)90496-7. [DOI] [PubMed] [Google Scholar]
- Takemoto L. J., Fox C. F., Jensen F. C., Elder J. H., Lerner R. A. Nearest-neighbor interactions of the major RNA tumor virus glycoprotein on murine cell surfaces. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3644–3648. doi: 10.1073/pnas.75.8.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto L. J., Miyakawa T., Fox C. F. Analysis of membrane protein topography of Newcastle disease virus and cultured mammalian fibroblasts. Prog Clin Biol Res. 1977;17:605–614. [PubMed] [Google Scholar]
- Teramoto Y. A., Puentes M. J., Young L. J., Cardiff R. D. Structure of the mouse mammary tumor virus: polypeptides and glycoproteins. J Virol. 1974 Feb;13(2):411–418. doi: 10.1128/jvi.13.2.411-418.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbrink F., Koornstra W., Bentvelzen P. The major polypeptides of the murine-mammary-tumor virus isolated by plant-lectin affinity chromatography. Eur J Biochem. 1977 Jun 1;76(1):85–90. doi: 10.1111/j.1432-1033.1977.tb11572.x. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J., Waterfield M. Evidence from studies with a cross-linking reagent that the haemagglutinin of influenza virus is a trimer. Virology. 1977 Jun 15;79(2):446–448. doi: 10.1016/0042-6822(77)90371-3. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L., Kaplan H. S. Structural characteristics of some murine RNA tumor viruses studied by lactoperoxidase iodination. Proc Natl Acad Sci U S A. 1973 Jan;70(1):36–40. doi: 10.1073/pnas.70.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]