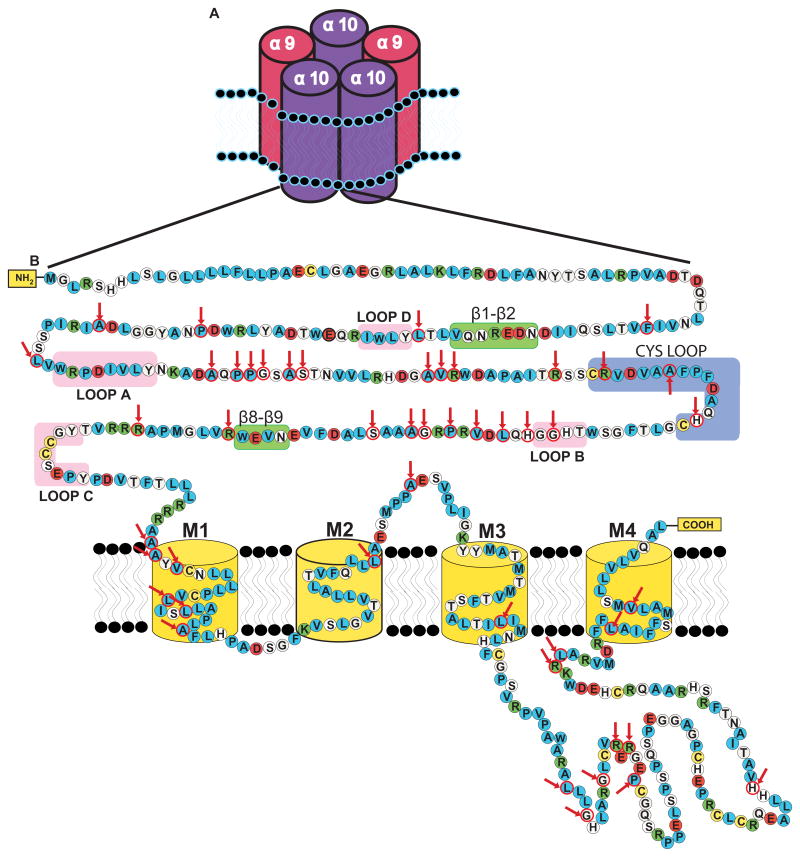
Figure 2.
(A) Schematic representation of the structure of the α9α10 nicotinic receptor, showing the arrangement of the subunits according to the predicted stoichiometry determined by Plazas et al. (2005). (B) Amino acid sequence and secondary structure of the human α10 receptor (the topology of the predicted α-helical structure of transmembrane regions are not reproduced). The properties of the amino acid side chains are indicated by different colors: polar (white), non-polar (blue), acidic (red), basic (green), and cysteine residues (yellow). Loops A, B, C, and D highlighted in pink correspond to regions involved in agonist binding. The Cys-loop (blue) and the β1–β2 and β8–β9 (green) correspond to regions involved in gating of the channel. Positive selected amino acids in mammals detected by the evolutionary analysis performed by Franchini and Elgoyhen (2006) are highlighted with red circles and indicated with red arrows.