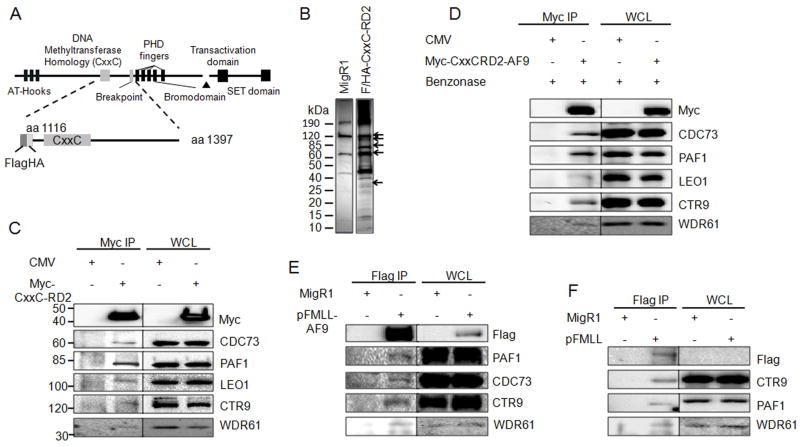
Figure 1. MLL binds to the PAF complex in a DNA independent manner.
A) Schematic diagram of the full length MLL protein with key domains indicated. The Flag/HA tagged CxxC-RD2 MLL fragment used for immunoprecipitation is shown below. The first and last amino acids of the protein fragments are indicated. B) MigR1 and Flag/HA-tagged CxxC-RD2 was expressed in 293 cells and immunoprecipitated. Immunoprecipitates were analyzed by SDS-PAGE and visualized by silver staining. Arrows indicate bands at the predicted molecular weights of the PAF complex components. C) Immunoprecipitation and western blot of the PAF complex following immunoprecipitation of Myc tagged CxxC-RD2 or Myc tag control from transiently tranfected 293 cells. D) The experiment described in C was repeated after treatment of the lysate with benzonase to digest DNA indicating the PAFc interaction with CxxC-RD2 is DNA-independent. Myc tagged CxxC-RD2-AF9 but not the Myc tag alone interacts with PAFc subunits showing that PAFc binding to CxxC-RD2 is maintained in the presence of the AF9 translocation partner. E and F) A full length flag tagged MLL-AF9 fusion protein (E) or Flag tagged full length MLL (F) was expressed in 293 cells and immunoprecipitated. The PAF complex stably associated with full length MLL fusion protein and MLL as indicated by western blot. (See also Figure S1)