Abstract
Aging has readily observable effects on the ability to resolve conflict between competing stimulus attributes that are likely related to selective structural and functional brain changes. To identify age-related differences in neural circuits subserving conflict processing, we combined structural and functional MRI and a Stroop Match-to-Sample task involving perceptual cueing and repetition to modulate resources in healthy young and older adults. In our Stroop Match-to-Sample task, older adults handled conflict by activating a frontoparietal attention system more than young adults and engaged a visuomotor network more than young adults when processing repetitive conflict and when processing conflict following valid perceptual cueing. By contrast, young adults activated frontal regions more than older adults when processing conflict with perceptual cueing. These differential activation patterns were not correlated with regional gray matter volume despite smaller volumes in older than young adults. Given comparable performance in speed and accuracy of responding between both groups, these data suggest that successful aging is associated with functional reorganization of neural systems to accommodate functionally increasing task demands on perceptual and attentional operations.
Keywords: Conflict, Stroop, Perceptual Cueing, Functional Reorganization, structural MRI, functional MRI
1. Introduction
A hallmark of aging is decline in selective brain functions that itself promotes neural reorganization for functional compensation (Cabeza et al., 2002; Reuter-Lorenz and Cappell, 2008; Ward, 2006). With aging, changes in perceptual and attentional functions require commensurate changes in strategies for effective selection of relevant and inhibition of irrelevant information (Schneider and Pichora-Fuller, 2000). Selection is particularly challenging for tasks with conflicting information, i.e., when an “automatic” response to overlearned task-irrelevant information needs to be inhibited for appropriate response selection. In this case, shrinking resources in older adults can make selection of task-relevant from irrelevant information especially challenging (Cohn et al., 1984; Gazzaley et al., 2005; Gazzaley et al., 2008; Kramer et al., 1994; Madden et al., 2004; Rabbitt, 1965; Raz, 2000). Processing of irrelevant and conflicting information may change in the course of aging and may depend on accommodation processes subserving and defining resource availability (Rajah and D'Esposito, 2005). In other words, age-related deficits in cognitive control that seem to be accelerated with increasing perceptual load may actually reflect the application of limited resources when processing distracting information (Madden and Langley, 2003).
Accordingly, it has been suggested that tasks involving high perceptual load that engage full attentional capacity in the processing of task-relevant stimuli leave no resources for the processing of any task-irrelevant stimuli, whereas tasks involving low perceptual load leave resources available for processing of irrelevant stimuli (Lavie, 1995). This trade-off can result in a paradox observed by Maylor and Lavie (1998): Older adults demonstrated greater interference under low (e.g., small number of nontargets) than high perceptual load conditions (e.g., a high number of nontargets). One interpretation was that under low load conditions, older adults had adequate resources to process multiple tasks, whereas a high perceptual load restricted resources for processing interfering information.
Moreover, repetitive conditions likely require less cognitive control and, in turn, may free up resources in the elderly for conflict resolution. For example, in young adults, stimulus-response repetitions reduced conflict measured behaviorally (Gratton et al., 1992; Mayr et al., 2003) and reduced activity in frontoparietal cortices measured with functional imaging (Egner and Hirsch, 2005; Kerns et al., 2004; Larson et al., 2009). This pattern of facilitation from repetition is preserved in older adults (Müller-Oehring et al., 2007; Soldan et al., 2008). The benefit from stimulus-response repetition appears independent of conscious awareness and may rely on a non-declarative implicit type of memory subserved by cortico-striatal brain circuits (Mishkin et al., 1984). However, the exact neural mechanisms, responsible for this progressive accommodation have not been investigated.
Functional neuroimaging studies have shown that conflict resolution entails the activation of both an anterior executive control system, involving anterior cingulate and prefrontal cortical circuitry associated with conflict detection and resolution (Harrison et al., 2005; MacDonald et al., 2000; Mayr et al., 2003), and a posterior attention system, involving the right parietal cortex associated with top-down attentional control on perceptual selection and stimulus attribute identification (Casey et al., 2000; Hazeltine et al., 2003; Milham et al., 2002). Thus, to examine whether processing advance perceptual information to resolve an impending conflict is unique to the anterior control system or extends to posterior attention and sensory systems, we devised a Stroop Match-to-Sample task that required matching the color of a cue stimulus to the color of a Stroop target stimulus (Schulte et al., 2009). The Stroop effect measures conflict between irrelevant words and relevant colors and is defined by a prolonged response by subjects asked to name the color type of a word printed in a color incongruent with the word's meaning (e.g., the word BLUE printed in red type) relative to when the word's meaning and color type are congruent (e.g., BLUE printed in blue type) (McLeod, 1991; Stroop, 1935).
Aging is associated with increases in activation during conflict processing in frontal (left inferior frontal gyrus, presupplementary motor areas) and parietal (intraparietal sulcus) brain areas (Braver and Barch, 2002; Langley et al., 2005; Milham et al., 2002), suggesting that additional engagement of both anterior and posterior attention systems may have compensated for age-related general decline (Grady 2008; Zysset at al., 2007). Such compensatory brain activity has been described as recruitment of an alternative network by older adults not normally used by young adults to enable comparable Stroop performance (e.g., Cabeza 2001; Stern et al., 2005). For example, in older adults not only has increased frontal activity been observed in conjunction with reduced occipitotemporal activity during a face and location perception task (Grady et al., 1994), but also increased posterior activation has been found in conjunction with reduced orbitofrontal activity during a delayed match-to-sample task (Lamar et al., 2004; Resnick et al., 2007).
By contrast, relatively little is known about the neural substrates of age differences in perceptual cueing that directs attention to task-relevant features in situations of cognitive conflict. In a visual search task, Humphrey and Kramer (1997) demonstrated that older and younger adults benefited to the same extent from the addition of a relevant feature when searching for targets defined by conjunctions of three features. Thus, valid cues can provide helpful information for processing of the upcoming conflict, and may activate the prefrontal and parietal cortices to employ preparatory strategy to maximize performance for conflict resolution (Schulte et al., 2005).
To assess attentional control processes and to be in the position to change test parameters affecting these processes, we employed a Stroop Match-to-Sample task in which a cue was presented that was either a valid or invalid predictor of the upcoming Stroop stimulus color and either guided or misguided attention (Schulte et al., 2005, 2006, 2008, 2009). Using this paradigm in healthy young adults, a functional MRI study revealed a dissociation of anterior and posterior attention systems for attentional control modulated by perceptual cueing (Schulte et al., 2009).
In the present study, we asked whether age-related differences in neural activation patterns during conflict processing depend on 1) repetition, i.e., when successive trials require the same responses, or 2) perceptual cueing, i.e., when the cue color matched or did not match the color of the Stroop target stimulus. We tested the following hypotheses: (1) Repetition of task demands would reduce behavioral Stroop conflict in both older and young adults and this would be associated with less activation in prefrontal and parietal cortices in both groups. By contrast, during non-repetitive Stroop conditions older adults would show greater engagement of a frontoparietal attention system than young adults. (2) When cue color matched Stroop stimulus color (Stroop-match), we predict similar Stroop accuracy performance in young and older adults. By contrast, when the cue color is invalid and does not match the Stroop stimulus color (Stroop-nonmatch), high perceptual processing demands would limit already reduced resources for processing the Stroop word's meaning in older adults and paradoxically reduce behavioral Stroop interference (cf., Schulte et al., 2009). Stroop-nonmatch processing will be associated with less activation in anterior `conflict processing' areas in both groups. (3) Finally, relationships between brain activity and gray matter volume in younger and older adults are explored to examine whether typical age-related decline in the volume of gray matter (e.g., Good et al., 2001; Kennedy and Raz, 2009; Pfefferbaum et al., 1994) is the principal contributor to age-related differences in activation patterns.
2. Methods
2.1 Subjects
The participants were 20 young and 19 older healthy adults. Of these, 6 subjects were excluded; 1 older woman pressed only one response button, 1 older man had more than a 31% error rate, and 4 subjects (3 older, 1 young) were excluded for head movements > 2mm during Stroop Match-to-Sample task performance in the MRI scanner. Thus, we analyzed functional brain and behavioral data of 19 young (mean age = 23.6 ± 3 years, range = 19–30) and 14 older (mean age = 71 ± 8.7 years, range = 58–85) healthy and normal-sighted adults. Groups did not differ in sex distribution (young: 10 women, 9 men; elderly: 6 women, 8 men; χ2 = 0.31, p = 0.58). All subjects were neurologically healthy, right handed (Crovitz, 1962; young: 19.2 ± 3.5; older adults: 17.8 ± 2.6; p = 0.26), had English as their first language and had no history of illicit substance or alcohol abuse or dependence according to DSM-IV criteria. Both groups were highly educated (young: 15.8 ± 1.1 years; older adults: 17.3 ± 2.7 years; p = 0.08). The young adults not having completed their education can explain the trend toward significant group difference in education level, as most of the young adults plan to go to graduate schools.
From the 14 older adults, a medical history indicated that one woman was taking a beta-blocker (Atenolol) and two men and two women were taking cholesterol-regulating medications (Lovastatin, HCT, Simvastatin) prophylactically. All medications were taken at a stable dosage for at least the past year prior to the MRI scans. One older adult had received localized chemotherapy for bladder cancer treatment two years prior to the study. Older adults were recruited from two ongoing studies of normal aging and scored well on the dementia screening tests used in each study: Dementia Rating Scale (Mattis, 1988), n = 9, mean = 141.8, range = 139–144 out of 144, Mini-Mental State Examination (Folstein et al., 1975), n = 4, mean = 28.2, range = 27–29 out of 30. Reduced visual function in older individuals may influence the performance on the Stroop Color-Word Test and scores are likely to be underestimated in individuals with low visual acuity (van Boxtel et al., 2001). To address this, we assessed visual acuity and contrast sensitivity in our young and older study participants using the Freiburg Visual Acuity and Contrast Test (FrACT), a computer program that uses psychometric methods based on the signal detection theory to estimate the acuity threshold (Bach 1996). Landolt-Cs were presented on a monitor in one of eight orientations. The subject pressed one of eight buttons, which are spatially arranged on a response box according to the eight possible positions of the Landolt-C's gap. As expected, older adults had poorer visual acuity (F(1,30) = 20.95; p < 0.0001) and contrast sensitivity (F(1,30) = 7.66; p < 0.01) than young adults. Subjects gave written informed consent to participate in brain imaging studies that were approved by the Institutional Review Boards at Stanford University School of Medicine and SRI International.
2.2 Data acquisition and analyses
Stroop Match-to-Sample Task
Stimuli were created and presented with PsyScope software. Subjects matched the color of a cue stimulus displayed for 450 ms in the center of the screen to the color of a Stroop target stimulus that appeared for 1100 ms after an interstimulus interval of 300 ms. Cue and target colors were either red, green or blue. Total trial duration was 3.3 sec. The color cue either matched or did not match the color of the Stroop target, which was either congruent (word blue written in blue ink) or incongruent (word blue written in red type). The Stroop effect is defined as the difference in reaction time (RT) to incongruent and congruent stimuli. In incongruent-nonmatch conditions the cue's color always matched the word's content (e.g., red cue, word RED written in green type). To accommodate the poorer visual ability in older adults, we used large size letters for all subjects regardless of age with high visual contrast for display of cue and Stroop stimuli in the MRI scanner.
All subjects performed a practice trial before entering the scanner. Prior to task performance in the scanner, instructions were reviewed via the scanner intercom system. Subjects used index and middle fingers of their dominant hand to press a YES-key for cue-target color matches and a NO-key for nonmatches, yielding accuracy and RT measures (Figure 1). To mix YES- and NO responses four blocks were presented, two containing incongruent match and non-match trials (incongruent, INC) and the other two containing congruent match and nonmatch trials (congruent, CON) in addition to four same-response blocks (congruent-match, congruent-nonmatch, incongruent-match, incongruent-nonmatch) (Figure 1). Trials presented in same- and mixed-response blocks were the same, only the order of trials differed. Total number of trials was 96 per run. Two runs were presented with 18 blocks each (1 block = 9 TRs or 6 trials; TR = 2.2 sec) including two rest condition blocks at the end of each run (12 trials per run). In the rest condition, subjects passively viewed the word `REST' in different colors (red, green, blue) presented for the same trial duration than Stroop match-to-sample trials. The start of the scan was triggered automatically by PsyScope software. Test instructions were reviewed with the subject by the examiner in a practice session of 32 trials before entering the scanner and again through the scanner's intercom system before the onset of each run. Subjects had a short break of ~ 6 minutes, between run 1 and run 2, but remained in the scanner.
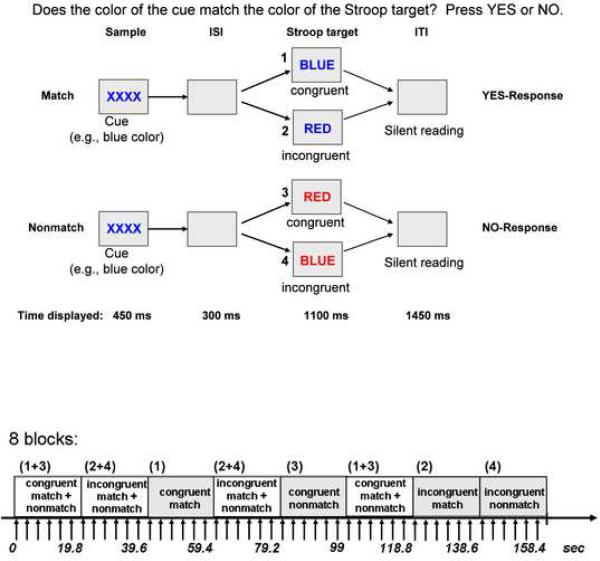
Figure 1.
Top: Stroop Match-to-Sample design, illustrating 4 conditions: incongruent-match, congruent-match, incongruent-nonmatch, and congruent-nonmatch. A color cue (XXXX) presented for 450ms was followed by an incongruent or congruent Stroop target stimulus that appeared for 1100ms after an inter-stimulus interval (ISI) of 300ms. The inter-trial interval (ITI) was 1450 ms. Subjects matched the color (red, green or blue) of the cue to the ink color of the Stroop stimulus. Bottom: fMRI block design illustrated for 8 blocks. Each block consisted of 6 trials (9 TRs). Each block lasted for of 19.8 sec. Stroop stimuli in each block were either congruent (word BLUE written in blue font) or incongruent (word BLUE written in red font). In half of the blocks cue-target color either matched or did not match, in the other half of the block match and nonmatch trials were mixed. In total 36 blocks were presented in pseudo-random order ensuring that each condition was equally often represented.
For behavioral data analysis, repeated measures analysis of variance (ANOVA) was used to test for group effects (young, old) as between subjects' factor and to test for Stroop effects (incongruent, congruent), perceptual cueing (color nonmatch, color match) and repetition effects (mixed response block, same response block) as within subject's factors. The interaction term from a 4-way ANOVA tested whether perceptual cueing effects on Stroop processing differently affected repetitive and non-repetitive trials and whether these effects were modulated by age. Derivative 2-way interactions identified the significantly influential variables and the extent of their effect. Significant interactions were followed up with t-tests. Significant levels were set at p = 0.05, two-tailed (SPSS 16.0).
fMRI Acquisition
Imaging was performed with a 3.0-T whole body MRI scanner (General Electric Medical Systems, Signa, Waukesha, WI, USA) using the Array Spatial Sensitivity Encoding Technique (ASSET) 3T head coil. Structural MRI protocols consisted of a spin-echo localizer scan and a T2-weighted fast spin-echo anatomical scan (axial acquisition; TE = 17 ms; TR = 5000 ms; FOV = 24 cm; 256 × 192 matrix; NEX = 1.0; 5 mm slice thickness; 0 mm skip; 36 slices) used for spatially registering the fMRI data. In addition, a standard 3D T1-weighted inversion recovery fast-spoiled gradient-recalled (SPGR) sequence (axial acquisition; TE = minimum; Prep time = 300 ms; flip angle = 15, bandwidth = 31.25 kHz; FOV = 24 cm; 256 × 256 matrix, NEX = 2.0; slice thickness = 1.25 mm; voxel dimension = 0.9375 × 0.3975 × 1.25 mm; 124 slices) was acquired in 15 younger and 12 older healthy study participants. Whole-brain fMRI data were acquired with a T2*-weighted gradient echo planar pulse sequence (axial, mode = 2D; scan timing: TE = 30 ms; TR = 2200 ms; flip angle = 90°; matrix = 64 × 64; voxel dimension = 3.75 × 3.75 × 5 mm; 36 slices). Image preprocessing and statistical analyses were performed using the SPM2 software package (Wellcome Department of Cognitive Neurology, University College London, UK).
fMRI Analysis
The functional images were subjected to motion correction, and the T2-weighted FSE structural images were coregistered to the motion-corrected functional mean images for each subject. All data were inspected for movement artifacts and did not exceed 2 mm (group mean±SD for the elderly was 1.18 mm±0.34 mm, and for the young 0.76 mm±0.27 mm, t(31) = 3.91, p < 0.0001). The images were then normalized to MNI (Montreal Neurological Institute, Quebec, Canada) space, and the volumes were smoothed with a Gaussian kernel of 8 mm (FWHM). Individual statistics were then computed using a general linear model approach (Friston et al., 1995) as implemented in SPM2. Statistical preprocessing consisted of high pass filtering at 39.6s, low pass filtering through convolution with the SPM2 canonical hemodynamic response function and global scaling. Global intensity values for EPI sequences after normalization and global scaling did not significantly differ between groups (t(31) = 0.68; p = 0.49).
A random effect analysis was conducted for group averaging and population interference, where one image per contrast was computed for each subject, and these images were subjected to t-tests, which produced a statistical image for the following contrasts for each subject: Stroop (INC > CON) for mixed response blocks, for same response blocks, for cue-target match and cue-target nonmatch conditions; incongruency (INC) for nonmatch versus match (NM > M), and congruency (CON) for nonmatch versus match (NM > M) conditions. For second level (group) analyses, these contrasts for each individual were entered in one- and two-sample t-tests. Analyses were carried out with an uncorrected P value threshold of 0.001, and k = 10 voxels as extent threshold.
We additionally tested whether our findings were robust when using a threshold that corrects for multiple comparisons. Accordingly, we used a statistical threshold with a joint-expected probability of p = .01 for height and p = .05 for extent corrected for the whole brain (Poline et al. 1997). We further tested whether our findings were robust when using head movement as a covariate and found no evidence that head motion significantly influenced BOLD signal intensity differences between young and older adults for each contrast of interest (Tables 2–4). For display purposes, group activations were superimposed onto a single subject T2-weighted SPM2-template image. Brain areas were determined by using the MNI coordinate function in MRICro, Version 1.40, from Chris Rorden (http://www.mricro.com). For validation, SPM-MNI coordinates in tables were transformed into the coordinate system of the Talairach and Tournoux (1988) stereotaxic atlas using the transformation from Matthew Brett (http://www.mrc.cbu.cam.ac.uk/Imaging/mnispace.html). Activations in the cerebellum were characterized using the atlas of Schmahmann et al. (2000).
Table 2.
Comparison of young and older adults: 1) Functional MRI (fMRI): Activity of brain regions preferentially invoked in each group for Stroop (INC-CON) contrasts for mixed and same response blocks. Coordinates are reported as given by SPM2 (MNI space) and correspond only approximately to Talairach and Tournoux space. Z = Z-value, two sample t-test (p < 0.001 uncorrected, extent threshold k = 10 voxels); BA = Brodmann area; kE = number of voxels in a cluster; 2) Voxel-based morphometry (VBM): gray matter volumes for regions of interest (ROIs), MANOVA, regions significant at p < 0.05 (SPSS); 3) Activity of brain regions after gray matter volume correction, ANCOVA
| fMRI | VBM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MNI-coordinates | Gray matter | ||||||||
| Brain region | BA | kE | t | Z | Older < Young | ||||
| X | Y | Z | F | p | |||||
| Stroop - Mixed Response Blocks: | |||||||||
| Older > Young | |||||||||
|
| |||||||||
| R. precuneus | 23 | 62 | 22 | − 56 | 28 | 4.391 | 3.84 | 0.25 | 0.62 |
| R. medial superior frontal gyrus | 9 | 11 | 12 | 40 | 54 | 4.361 | 3.82 | 29.8 | 0.0001 |
| R. anterior and middle cingulate cortex | 24 | 61 | 8 | 12 | 24 | 4.241 | 3.74 | 6.45 | 0.018 |
| 8 | 2 | 28 | 4.211 | 3.71 | |||||
| L. anterior, middle cingulate cortex | 23 | 23 | − 6 | − 6 | 30 | 4.181 | 3.69 | 5.46 | 0.028 |
| R. insula | 48 | 31 | 42 | 4 | 4 | 4.011 | 3.57 | 34.8 | 0.0001 |
|
| |||||||||
| Young > Older | |||||||||
|
| |||||||||
| no suprathreshold voxels | |||||||||
|
| |||||||||
| Stroop - Same Response Blocks: | |||||||||
| Older > Young | |||||||||
|
| |||||||||
| L. calcarine sulcus | 17/18 | 48 | − 4 | − 66 | 10 | 3.93 | 3.51 | 48.0 | 0.0001 |
| L. superior parietal lobe, precuneus | 5 | 14 | − 14 | − 46 | 76 | 3.841 | 3.45 | 30.5 | 0.0001 |
| L. paracentral gyrus, precuneus | 4 | 27 | − 6 | − 38 | 70 | 3.771 | 3.40 | 35.2 | 0.0001 |
|
| |||||||||
| Young > Older | |||||||||
|
| |||||||||
| R. insula | 48 | 47 | 40 | 2 | 2 | 3.95*1 | 3.53 | 43.2 | 0.0001 |
| R. middle frontal gyrus | 46 | 21 | 40 | 42 | 26 | 3.821 | 3.43 | 5.54 | 0.011 |
| L.insula | 48 | 10 | − 42 | − 4 | − 6 | 3.741 | 3.37 | 38.6 | 0.0001 |
regions significant at p < 0.05 corrected for the whole brain
regions significant at p < 0.05 corrected for regional gray matter volumes (SPSS).
Table 4.
Comparison of young and older adults: 1) Functional MRI (fMRI): Activity of brain regions preferentially invoked in each group for (A) incongruent match versus nonmatch and (B) congruent match versus nonmatch trials. Coordinates are reported as given by SPM2 (MNI space) and correspond only approximately to Talairach and Tournoux space. Z = Z-value, two sample t-test (p < 0.001 uncorrected, extent threshold k = 10 voxels); BA = Brodmann area; kE = number of voxels in a cluster; 2) Voxel-based morphometry (VBM): gray matter volumes for regions of interest (ROIs), MANOVA, regions significant at p < 0.05, two-tailed (SPSS); 3) Activity of brain regions after gray matter volume correction, ANCOVA
| fMRI | VBM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MNI-coordinates | Gray matter | ||||||||
| Brain region | BA | kE | t | Z | Older < Young | ||||
| X | Y | Z | F | p | |||||
| (A) Incongruent-Nonmatch versus Incongruent-Match: | |||||||||
| Older > Young | |||||||||
|
| |||||||||
| R. middle and superior frontal gyri | 9, 46 | 28 | 24 | 42 | 32 | 4.331 | 3.80 | 12.1 | 0.002 |
| R. supramarginal gyrus, inferior parietal lobe | 40 | 129 | 56 | − 44 | 36 | 4.251 | 3.75 | 16.2 | 0.0001 |
| 56 | − 42 | 46 | 3.561 | 3.24 | |||||
| L. middle frontal gyrus | 9, 46 | 66 | − 34 | 40 | 36 | 4.181 | 3.70 | 18.4 | 0.0001 |
| R. inferior frontal gyrus – pars opercularis, and insula | 45, 47, 48 | 32 | 44 | 14 | 4 | 4.111 | 3.65 | 29.9 | 0.0001 |
| L. superior frontal gyrus | 6 | 12 | − 20 | 20 | 64 | 3.641 | 3.29 | 31.6 | 0.0001 |
|
| |||||||||
| Young > Older | |||||||||
|
| |||||||||
| L. cerebellum – vermis and cerebellum 8, 9 | 47 | − 2 | − 72 | − 38 | 3.921 | 3.50 | 0.62 | 0.44 | |
| − 8 | − 64 | − 44 | 3.801 | 3.41 | |||||
| L. fusiform, parahippocampal and lingual gyri | 30, 37 | 20 | − 22 | − 40 | − 12 | 3.881 | 3.48 | 12.2 | 0.002 |
|
| |||||||||
| (B) Congruent-Nonmatch versus Congruent-Match: | |||||||||
| Older > Young | |||||||||
|
| |||||||||
| no suprathreshold voxels | |||||||||
|
| |||||||||
| Young > Older | |||||||||
|
| |||||||||
| L. inferior parietal lobe | 40, 7, 39 | 433 | − 36 | − 58 | 52 | 5.57*1 | 4.60 | 60.0 | 0.0001 |
| − 44 | − 48 | 48 | 3.78*1 | 3.40 | |||||
| R. supramarginal gyrus, inferior parietal lobe | 40 | 109 | 56 | − 46 | 42 | 4.831 | 4.14 | 30.8 | 0.0001 |
| L. precentral gyrus | 6 | 228 | − 58 | 10 | 32 | 4.24*1 | 3.74 | 27.6 | 0.0001 |
| − 40 | 2 | 40 | 3.73*1 | 3.37 | |||||
| L. middle frontal gyrus | 46 | 153 | − 36 | 24 | 42 | 4.23*1 | 3.73 | 5.46 | 0.028 |
| L. middle frontal gyrus | 45, 46 | 28 | − 36 | 46 | 18 | 3.67*1 | 3.32 | 3.12 | 0.089 |
| − 38 | 42 | 28 | 3.41*1 | 3.12 | |||||
| L. middle, superior frontal gyrus | 6, 8 | 18 | − 28 | 4 | 64 | 3.65*1 | 3.30 | 25.0 | 0.0001 |
regions significant at p < 0.05 corrected for the whole brain
regions significant at p < 0.05 corrected for regional gray matter volumes (SPSS).
For graphical illustration and display purposes (Figures 2–4), we used the MarsBaR regions-of-interest (ROI) analysis toolbox (marsbar.source-forge.net/) (Brett et al., 2002) to extract BOLD signals from significant clusters in the SPM functional MRI results. For each cluster, mean signal intensity values were extracted for each subject. Group means and standard errors of extracted BOLD signal intensities were calculated for each cluster for graphical illustration. For example, for the Stroop contrast `incongruent minus congruent,' positive group mean signal intensity values stand for more BOLD signal in incongruent than congruent conditions and negative values for less BOLD signal in incongruent than congruent conditions. The graphs illustrate the signal intensity values for each group underlying the General Linear Model (GLM) statistics in the SPM result tables.
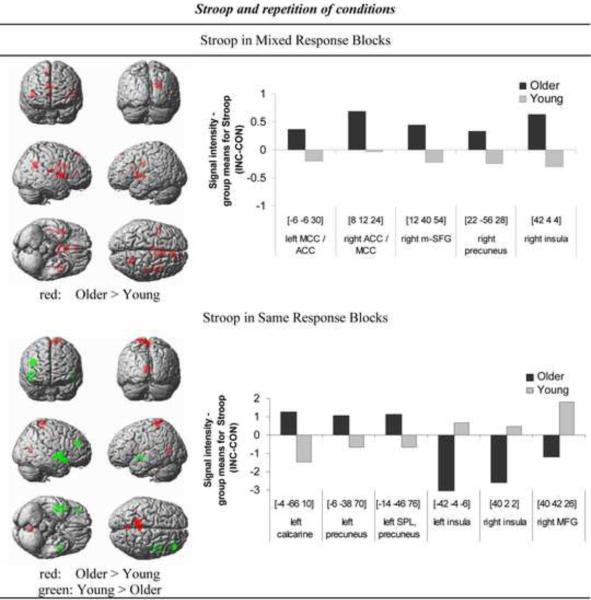
Figure 2. Stroop interference and condition repetition.
Brain regions demonstrating age differences in Stroop-related activation in mixed response blocks (upper panel) and same response blocks (lower panel). Regions in red reflect greater blood oxygen level-dependent (BOLD) response in older than young adults, and regions in green reflect greater BOLD response in young than older adults. The threshold has been lowered to P < 0.005 uncorrected for display purpose. Bar graphs on the right are showing mean BOLD signal intensity differences extracted using MarsBaR (http://marsbar.sourceforge.net/) during Stroop (incongruent (INC) minus congruent (CON)) performance for older and young adults for regions showing group differences significant at P < 0.001 uncorrected (Table 2).
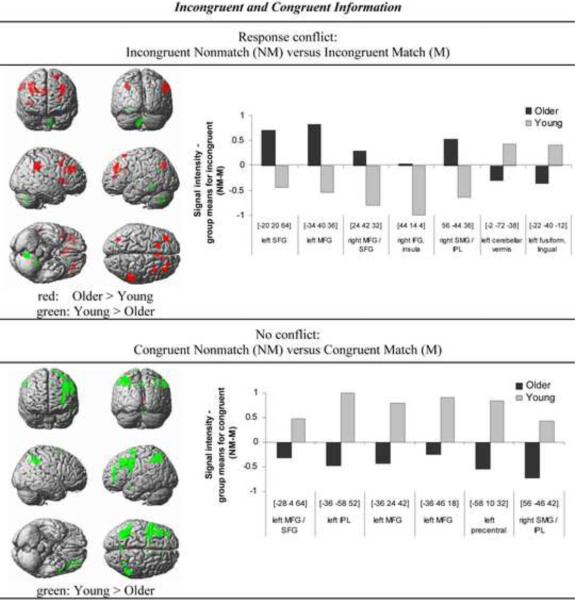
Figure 4. Response conflict, incongruent and congruent information.
Brain regions demonstrating age differences in response conflict-related activation (incongruent nonmatch – match) (upper panel) and non-conflict matching-related activation (congruent nonmatch – match) (lower panel). Regions in red reflect greater BOLD response in older than young adults, and regions in green reflect greater BOLD response in young than older adults. The threshold has been lowered to P < 0.005 uncorrected for display purpose. Bar graphs on the right are showing mean BOLD signal intensity differences extracted using MarsBaR (http://marsbar.sourceforge.net/) during cue-target color matching (nonmatch – match) for incongruent (upper panel) and congruent Stroop target stimuli (lower panel) in older and young adults for regions showing group differences significant at P < 0.001 uncorrected (Table 4).
MRI Analysis
For gray matter segmentation and volume extraction, we used high-resolution SPGR scans that were acquired in 13 of 14 older and 17 of 19 young adults. Some scans were discarded because of acquisition errors and poor image quality (2 young, 1 older). Structural SPGR sequence data were processed using SPM8 software (Wellcome Department of Cognitive Neurology, London) running on MATLAB version 7.7 (The MathWorks, Natick, MA). MRI data were analyzed using the optimized approach of voxel-based morphometry (VBM) (Good et al., 2001). VBM analysis includes the following steps: First, study-specific templates of gray and white matter were created for automated segmentation and spatial normalization of the initial images. These templates were created from the images of younger and older healthy subjects to ensure the data from both groups were treated equally during spatial normalization. All images were registered to the `T1' template of MNI (Montreal Neurological Institute) using an affine transformation (Ashburner and Friston, 2000) and then segmented into gray matter, white matter, and cerebrospinal fluid (CSF). Only the gray matter images were retained for subsequent processing. Gray matter images were then averaged and smoothed using an isotropic 8 mm-FWHM (full width at half maximum) Gaussian smoothing function.
Subsequent image processing included the following steps: First, the native MRI scans were segmented and gray matter images nonlinearly transformed with the study-specific gray matter template to derive the normalization parameters subsequently applied to the initial images. This step allowed optimal spatial normalization of gray matter to the customized gray matter template and reduced the contribution of any non-brain voxels. Second, these native images were then resampled with third-order B-spline interpolation to a final voxel size of 1.5×1.5×1.5mm3. Third, the normalized native images were segmented into gray matter, white matter, and CSF. Fourth, a Jacobian modulation was applied by multiplying the voxel intensities by the Jacobian determinants derived from the nonlinear component of the spatial normalization step. This step compensated for the voxel volume modification induced by nonlinear spatial normalization (Good et al, 2001). Finally, the modulated images from younger and older subjects were smoothed with an 8 mm-FWHM isotropic Gaussian smoothing function. After smoothing, each voxel represents the local average amount of gray matter in the surrounding region, the size of which is determined by the smoothing kernel. For whole-brain statistical analyses of regional gray matter volume differences between young and older adults significance levels were set at P < 0.025, FWE corrected, for one-tailed t statistics (Good et al., 2001). For correlation analyses with functional activation data, we used the MarsBaR regions-of-interest (ROI) analysis toolbox (Brett et al., 2002) to extract mean gray matter signal intensity values for each subject for those clusters showing significant group differences in activation (Tables 2–4). Furthermore, to test whether functional activation differences between young and older adults could be attributed to anatomical differences, we used covariance analyses (ANCOVA) with gray matter volumes as covariates for group-related BOLD-signal differences for each contrast of interest.
3. Results
3.1 Behavioral results
Error Analysis
Incidence of errors was less than 2.5% (mean ± SD for all values; young: 3.3 ± 3.6; older adults: 4.3 ± 4.6) and misses less than 1% (young: 0.5 ± 1.6; older adults: 2.4 ± 6.6), indicating high accuracy levels by both groups while performing the Stroop Match-to-Sample task in the scanner. The groups did not significantly differ in errors (F(1,31) = 0.45, p = 0.51), misses (F(1,31) = 1.47, p = 0.24), or overall reaction time (RT) (F(1,31) = 0.85, p = 0.36; MANOVA). Moreover, Stroop performance measures were not related to individual's visual acuity and contrast sensitivity (all r < 0.36; p > 0.1).
Reaction Time Analysis
A repeated measures ANOVA with group (young, older adults) as between-subjects factor and Stroop effect (incongruent, congruent), match (nonmatch, match), and response block (mix, same) as within-subject factors revealed a significant interaction among all four factors: group-by-Stroop-by-match-by-response block interaction (F(1,31) = 7.85, p < 0.009). Stroop effects were less robust in older than young adults (group-by-Stroop interaction; F(1,31) = 32.2, p < 0.025) (see also Table 1). As expected, Stroop effects were larger in match than nonmatch trials (Stroop-by-match interaction, F(1,31) = 5.29, p = 0.028) but did not differ between same and mixed response blocks (Stroop-by-response block interaction, F(1,31) = 1.21, p = 0.28). We further found significant main effects for Stroop with longer RTs to incongruent than congruent trials (F(1,31) = 32.2, p < 0.0001), for match (i.e., perceptual cueing) with longer RTs to nonmatch than match trials (F(1,31) = 7.72, p < 0.009), and for response block (i.e., repetition) with longer RT to trials in mixed than same response blocks (F(1,31) = 12.7, p < 0.001).
Table 1.
Condition reaction time means for young and older adults
| Over All Conditions | Young | Older | ANOVA |
|---|---|---|---|
| Congruent | 800.7 (56.7) | 747.3 (43.7) | |
| Incongruent | 863.3 (59.6) | 773.1 (46.8) | |
| Stroop (incongruent – congruent) | 62.7 (9.5) | 25.9 (12.9) | F = 5.56, p = 0.025 |
| t Test | t = 6.63, p = 0.0001 | t = 2.01, p = 0.066 |
| Mixed Response Blocks Only | |||
|---|---|---|---|
| Congruent | 800.3 (54.7) | 765.8 (45.1) | |
| Incongruent | 872.4 (59) | 796.1 (47) | |
| Stroop (incongruent – congruent) | 72.1 (14.6) | 30.3 (10.6) | F = 4.68, p = 0.038 |
| t Test | t = 4.93, p = 0.0001 | t = 2.87, p = 0.013 |
| Same Response Blocks Only | |||
|---|---|---|---|
| Congruent | 801.1 (59) | 728.7 (43.1) | |
| Incongruent | 854.3 (60.9) | 750.2 (47.9) | |
| Stroop (incongruent – congruent) | 53.2 (10.7) | 21.5 (19.4) | F = 2.33, p = 0.14 |
| t Test | t = 4.96, p = 0.0001 | t = 1.11, p = 0.29 |
| Match Trials Only | |||
|---|---|---|---|
| Congruent | 773.8 (61.3) | 692.8 (42.2) | |
| Incongruent | 844.4 (66.8) | 748 (50.3) | |
| Stroop (incongruent – congruent) | 70.7 (18.4) | 55.3 (27.4) | F = 0.24, p = 0.63 |
| t Test | t = 3.85, p = 0.001 | t = 2.01, p = 0.065 |
| Nonmatch Trials Only | |||
|---|---|---|---|
| Congruent | 828.5 (58.6) | 764.6 (46.6) | |
| Incongruent | 864.2 (56.1) | 752.3 (48.2) | |
| Stroop (incongruent – congruent) | 35.7 (14.7) | − 12.3 (22.5) | F = 3.47, p = 0.072 |
| t Test | t = 2.43, p = 0.026 | t = 0.55, p = 0.59 |
| Incongruent Trials Only | |||
|---|---|---|---|
| Match | 844.44 (66.8) | 748 (50.3) | |
| Nonmatch | 864.2 (56.1) | 752.3 (48.2) | |
| Match effect (nonmatch – match) | 19.8 (19.3) | 4.3 (22.8) | F = 0.27, p = 0.61 |
| t Test | t = 1.03, p = 0.32 | t = 0.19, p = 0.86 |
| Congruent Trials Only | |||
|---|---|---|---|
| Match | 773.8 (61.3) | 692.8 (42.2) | |
| Nonmatch | 828.5 (58.6) | 764.6 (46.6) | |
| Match effect (nonmatch – match) | 54.7 (21.2) | 71.8 (21.3) | F = 0.31, p = 0.58 |
| t Test | t = 2.58, p = 0.019 | t = 3.37, p = 0.005 |
Values in parentheses are SE.
Table 1 summarizes the results of follow-up F- and t-tests on Stroop effects (incongruent, congruent) for mixed and same response blocks, for cue-target color match and nonmatch trials, and for Match effects (nonmatch, match) for incongruent and congruent trials. Specifically, ANOVAs tested the study's hypotheses of repetition and perceptual cueing effects on Stroop processing in aging revealed the following results:
Stroop and condition repetition in aging
Older adults were less affected by Stroop interference than younger adults in mixed-response blocks (p = 0.038) but did not differ from young adults in same-response blocks (p = 0.14).
Stroop and perceptual cueing in aging
Stroop effects for match trials did not differ between groups (p = 0.63), but for nonmatch trials showed a trend for smaller Stroop effects in older than younger adults (p = 0.072). We further tested for perceptual cueing effects separately in incongruent and congruent trials, i.e., color matching (nonmatch – match) for incongruent and congruent information: Group differences were not forthcoming for either, incongruent (p = 0.61) or congruent trials (p = 0.58). Older and younger adults both profited from valid color cueing in congruent trials (young: 54.7 ms; older: 71.8 ms) but not in incongruent trials (young: 19.8 ms; older: 4.3 ms).
3.2 Neural correlates of Stroop Match-to-Sample effects
To localize brain areas that were more active during incongruent than congruent Stroop target processing, we generated Stroop contrast images (INC > CON) for each subjects. Stroop contrast images were computed for mixed and same response blocks and for match and nonmatch trials. For second-level group analyses, these Stroop contrast images were entered into two-sample t-tests for group comparison.
3.2.1 Stroop and condition repetition in aging
1. Stroop-mix contrast
During Stroop processing (INC > CON) in mixed response blocks, the elderly showed greater activation than the young in frontoparietal brain regions, including the precuneus (right BA 23), medial superior frontal gyrus (right BA 9), anterior and middle cingulate cortices (bilateral BA 23, 24), and the insula (right BA 48) (Table 2). Younger adults did not show greater activation than older adults when processing Stroop in mixed response blocks.
2. Stroop-same contrast
During Stroop processing (INC > CON) in same response blocks, older compared to younger adults activated occipitoparietal visuomotor brain regions including the striate cortex (left BA 17, 18), superior parietal lobe, precuneus (left BA 5) and paracentral brain regions (left BA 4). Relative to older adults, younger adults activated more frontal areas, including the insula (bilateral BA 48) and the middle frontal gyrus (right BA 46) (Table 2).
3.2.2 Stroop and perceptual cueing in aging
1. Stroop-match contrast
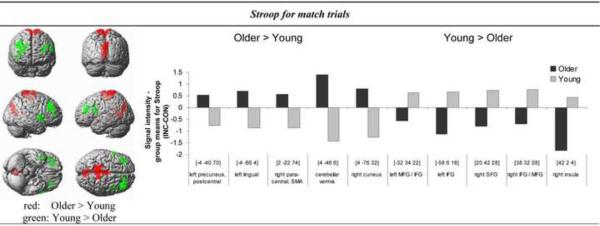
During Stroop processing (INC > CON) with valid pretrial color cueing (match), older adults showed greater BOLD response than young subjects in the posterior visual and motor brain areas including para- and postcentral gyri, precuneus, supplementary motor area (left BA 1, 5, bilateral BA 4), cerebellar vermis, lingual gyrus (left BA 18), and cuneus (right BA 18). By contrast, younger adults showed greater activation than the elderly in frontal brain regions, including bilateral inferior frontal cortex (BA 46), pars triangularis (BA 45) and pars opercularis (left BA 44), middle and superior frontal gyris (right BA 9), and insula (right BA 48) (Table 3).
Table 3.
Comparison of young and older adults: 1) Functional MRI (fMRI): Activity of brain regions preferentially invoked in each group for Stroop (INC-CON) contrasts for cue-target match or nonmatch trials. Coordinates are reported as given by SPM2 (MNI space) and correspond only approximately to Talairach and Tournoux space. Z = Z-value, two sample t-test (p < 0.001 uncorrected, extent threshold k = 10 voxels); BA = Brodmann area; kE = number of voxels in a cluster; 2) Voxel-based morphometry (VBM): gray matter volumes for regions of interest (ROIs), MANOVA, regions significant at p < 0.05 (SPSS); 3) Activity of brain regions after gray matter volume correction, ANCOVA
| fMRI | VBM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MNI-coordinates | Gray matter | ||||||||
| Brain region | BA | kE | t | Z | Older < Young | ||||
| X | Y | Z | F | p | |||||
| Stroop-Match: | |||||||||
| Older > Young | |||||||||
|
| |||||||||
| L. precuneus, paracentral and postecentral gyri | 5 | 226 | −4 | −40 | 70 | 4.56* | 3.96 | 39.4 | 0.0001 |
| 1 | − 12 | − 44 | 74 | 4.21* | 3.71 | ||||
| 4 | − 16 | − 34 | 76 | 3.60* | 3.27 | ||||
| R. paracentral gyrus, supplementary motor area | 4 | 26 | 2 | − 22 | 74 | 3.92* | 3.50 | 36.0 | 0.0001 |
| L. lingual gyrus | 18 | 132 | − 4 | − 66 | 4 | 4.43*1 | 3.87 | 51.5 | 0.0001 |
| R. cerebellum - vermis | 68 | − 4 | − 46 | 6 | 4.31*1 | 3.79 | 33.5 | 0.0001 | |
| R. cuneus | 18 | 97 | 4 | − 76 | 32 | 409*1 | 3.63 | 48.7 | 0.0001 |
|
| |||||||||
| Young > Older | |||||||||
|
| |||||||||
| R. middle and inferior frontal gyri | 46 | 178 | 38 | 32 | 28 | 5.36*1 | 4.48 | 1.12 | 0.30 |
| - pars triangularis | 9 | 36 | 32 | 42 | 3.91*1 | 3.50 | |||
| R. superior frontal gyrus | 9 | 63 | 20 | 42 | 28 | 4.62*1 | 4.00 | 0.52 | 0.48 |
| R. insula | 48 | 67 | 42 | 2 | 4 | 4.601 | 3.98 | 38.9 | 0.0001 |
| L. middle and inferior frontal gyri - pars triangularis | 46, 45 | 25 | − 32 | 34 | 22 | 3.85*1 | 3.45 | 0.05 | 0.83 |
| L. inferior frontal gyrus - pars opercularis | 44 | 11 | − 58 | 6 | 18 | 3.59*1 | 3.26 | 25.8 | 0.0001 |
|
| |||||||||
| No significant group differences for Stroop-Nonmatch activation | |||||||||
regions significant at p < 0.05 corrected for the whole brain
regions significant at p < 0.05 corrected for regional gray matter volumes (SPSS).
2. Stroop-nonmatch contrast
No group differences were observed for Stroop processing (INC > CON) with invalid pretrial color cueing (nonmatch).
3. Incongruent Stroop information, nonmatch-match (invalid-valid cue) contrast
When processing incongruent information older adults showed greater BOLD signal than the young group for nonmatch relative to match trials in frontoparietal brain areas, including middle and superior frontal gyri (bilateral BA 9, 46), inferior frontal gyrus (right BA 45, 48), insula (BA 48), supramarginal gyrus (right BA 40) and superior frontal gyrus (left BA 6). By contrast, younger subjects showed more activation than elderly subjects in some posterior cortices, including the junction between fusiform, lingual and parahippocampal gyri (left BA 30, 37), and the left cerebellum (Table 4A).
4. Congruent Stroop information, nonmatch-match (invalid-valid cue) contrast
When processing congruent Stroop information, however, older adults did not show greater BOLD signal than younger subjects for nonmatch (invalid cues) than match (valid cues) congruent trials. By contrast, younger adults exhibited greater BOLD signal than older subjects in frontoparietal brain areas, including inferior parietal lobe (bilateral BA 40, left BA 7, 39), middle frontal gyri (bilateral BA 46, left BA 45), and superior frontal and precentral gyri (left BA 6, 8) (Table 4B).
3.3 Gray matter volumes in young and older adults
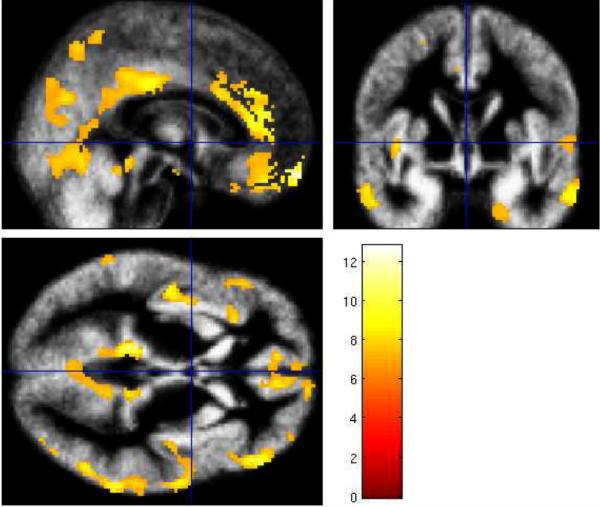
Whole-brain voxel-based morphometry (VBM, Good et al., 2001) revealed gray matter volume differences between young and older adults in several cortical regions (Figure 5): frontal cortices, anterior cingulate cortex and rectus (left: BA 11, 24, T=11.96), and inferior, middle and superior frontal gyri (left: BA 47, 48, T=9.21, BA 8, 9, T=8.84; right: BA 45, T=9.91); temporal cortices, inferior and middle temporal gyri (left: BA 21, 22, T=12.79, BA 27, 30, T=12.44, BA 36, T=9.36; right: BA 20, 21 T=9.96), and temporo-parietal junction (right: BA 40, 22, T=11.73); and parietal cortices, angular gyrus, superior parietal and inferior temporo-parietal regions (left: BA 39, 7, T=10.44), and middle cingulate cortex (right: BA 23, T=9.38). By contrast, in the occipital lobe only the left fusiform gyrus (BA 37, T=8.96) showed significant gray matter volume differences in young and older adults.
Figure 5.
VBM-detected gray matter volume differences (older < young adults) superimposed on the study-specific normalized mean group image from all subjects and presented in the sagittal, axial and coronal plane (T = 6.68, PFWE corrected = 0.025) (Good et al., 2001). The color bar represents the t scores.
Furthermore, voxel-based morphometry (VBM, Good et al., 2001) revealed significantly smaller gray matter volumes in older than younger subjects in most regions of interest (ROIs) derived from functional analyses (Tables 2–4). Group differences in functional BOLD-responses for contrasts of interest remained significant for most clusters after gray matter volume correction (significant at p < 0.05 corrected for regional gray matter volumes, SPSS) (Tables 2–4). BOLD responses in only one cluster in the `Stroop-same' contrast, i.e., the visual cortex (calcarine gyrus) (Table 2), and two clusters in the `Stroop-Match' contrast', i.e., bilateral motor association cortex (para-, postcentral gyri, supplementary motor area) (Table 3) were no longer significantly different between groups after gray matter volume correction. Within-groups correlation analyses between gray matter volumes and functional activation for each cluster demonstrated only one significant relationship in young adults and involved the left superior frontal gyrus. Here, larger gray matter volumes were related to more activity during nonmatch than match processing for incongruent trials (r = .52, p = 0.046, uncorrected for multiple comparisons). The volume-functional correlations in older adults were not significant.
4. Discussion
Structural and functional MRI identified age-related differences in neural circuits for conflict processing while healthy young and older adults performed a Stroop Match-to-Sample task involving perceptual cueing and repetition to manipulate task demands. Older adults showed comparable Stroop performance to younger adults but engaged a frontoparietal network more than young adults when processing non-repetitive conflict (Stroop mixed response block). Further, when processing conflict following a valid perceptual cueing (Stroop match), older adults engaged a visuomotor network more than young adults, whereas young adults activated frontal regions more than older adults. A separate analyses of perceptual cueing (nonmatch-match) for incongruent and congruent information revealed that with invalid perceptual cueing, i.e., when the cue's color did not match the Stroop target's type color but instead matched the incongruent word's content, older adults engaged a frontoparietal cognitive control network more and occipital brain regions less than younger adults. These differential activation patterns were not correlated with regional gray matter volume despite smaller volumes in older than young adults. These activation patterns differentiating young and older adults, in the context of overall high performance levels, suggest that older adults can efficiently adapt to task demands by the use of different strategies from young adults when resolving conflict.
4.1 Conflict adaptation to condition repetition in aging
Stroop processing in same and mixed response blocks
Neural correlates of Stroop processing differed between older and young adults as a function of condition repetition. When the same response was required repeatedly during Stroop conflict processing, older adults activated prefrontal areas and insula less and visual and motor association areas more than young adults. Low frontal activation is consistent with small behavioral Stroop effects (incongruent minus congruent reaction times) in older adults, and greater visuomotor activation may reflect improved response selection while matching cue to target colors (Kray et al., 2005). Thus, as the task becomes more repetitive, older adults use less executive attentional control systems and more visuomotor areas than young adults.
During Stroop processing in mixed response blocks, older adults showed greater activation than younger ones in midline frontoparietal brain regions, including the medial superior frontal gyrus, anterior and middle cingulate cortex, precuneus and insula, consistent with behavioral Stroop effects in older adults even though they were smaller than in young adults. Such a medial prefrontal-mid-parietal neural network has been previously suggested to be involved in internally guided attention (Cavanna and Trimble, 2006). The observed insula activation may reflect greater need in older than young adults to integrate sensory and control systems during perceptual color matching in Stroop conflict processing (Downar et al., 2001; Meehan and Staines, 2009). This difference is compatible with the idea that older adults recruit additional brain areas to those recruited by young adults for effective initiation of strategies to attenuate the Stroop effect (Langenecker et al., 2004; Zysset et al., 2007). The increased activity in midline frontoparietal brain areas in older adults during mixed response blocks suggests the use of higher-order cognitive control processes such as working memory (Byrne et al., 2007; Trinkler et al., 2009; Wallentin et al., 2006; Wagner et al., 2005) and conflict monitoring (Botvinick, 2007) to maintain color cue information for delayed matching and selecting the effective response. Similar to other studies on cognitive aging (Cabeza et al., 2002; Rosen et al., 2002), our high-functioning older adults may have engaged compensatory frontoparietal brain reserve specifically during Stroop processing in mixed response blocks to counteract age-related cognitive decline.
4.2 Conflict adaptation to perceptual cueing in aging
Stroop-match and Stroop-nonmatch
Stroop effects were greater when the color cue correctly predicted the color of the Stroop target (Stroop-match) than when it did not (Stroop-nonmatch) (cf., Schulte et al., 2008). Both groups profited from valid color cueing (match) especially in congruent trials. Behaviorally, the groups did not differ in either Stroop-match or Stroop-nonmatch performance but did differ in activation patterns of regional BOLD responses during Stroop-match. In the latter case, older adults activated a visuomotor network more and a prefrontal executive attention network less than young adults. Others also have found recruitment of visual processing regions in occipital and temporal cortices and inferior prefrontal regions in older than young adults during Stroop processing (Milham et al., 2002; Zysset et al., 2007), in contrast to several neuroimaging studies that have found decreases in the extent of activation in the occipital cortex in older relative to young adults (Grady et al., 1994; Langenecker et al., 2004; Madden et al., 1997, 2007; Paxton et al., 2008). It is possible that the different findings of occipital cortex activation between studies are due to the stimuli used (Kelley et al., 1998; Milham et al., 2002).
Alternatively, age-related differences in findings in occipital cortex activation may be explained by differences in the cognitive demands employed. To cope with these task demands and to compensate for deficits, older adults may utilize different neural circuits from young adults subserving and defining resource availability (compensation-related utilization of neural circuits hypothesis; CRUNCH, Reuter-Lorenz and Cappell, 2008; Reuter-Lorenz and Lustig, 2005), which implicates both overrecruitment (Li et al., 2001) and underrecruitment of brain areas in older adults (Schneider-Garces et al., 2009). However, group differences in activation were not present in the Stroop-nonmatch contrast. This is consistent with recent functional MRI findings that young healthy adults engaged only few regions of a predominantly posterior network during Stroop-nonmatch processing (Schulte et al., 2009). Older adults in the current study, however, activated a posterior network during Stroop-match processing more than the young. Thus, the lack of group difference in brain activation during perceptual cueing of the interfering feature in Stroop-nonmatch may reflect the fact that both young and older adults experienced high perceptual processing demands, which may limit resources to process incongruent information.
Consistent with CRUNCH, our data indicate that the activation of neural networks in high-functioning older adults is demand-specific, as evidenced by greater engagement of a frontoparietal network when the task involved top-down control (Stroop processing in mixed response blocks) and of a visuomotor network when the task involved perceptual cueing (Stroop-match processing). The different activation patterns seen in older adults and young adults during Stroop-match processing may reflect compensatory strategies and increased sensitivity to the presence of competing color information in the elderly. Alternatively, it is possible that older adults in our study processed incongruent and congruent trials similarly and focused on visual matching for motor response preparation (Melcher and Gruber, 2006; Milham and Banich, 2005), which raises the question of how older adults processed color-word incongruency.
4.2.1 Perceptual cueing of incongruent and congruent color-word conditions
When processing incongruent-nonmatch trials in contrast with incongruent-match trials, older adults elicited greater lateral frontoparietal attentional control regions and fewer occipital regions (fusiform) than the young. Similarly, Madden and colleagues (2007) found frontoparietal activation in older adults but occipital activation (fusiform) in young adults while performing a task requiring top-town attentional control. Activation of frontoparietal regions is typically associated with conflict resolution (Davelaar, 2008; MacDonald et al., 2000) and may have further facilitated selection of an appropriate response in the older adults by maintaining sensory material (cue's color) (Mars et al, 2008). By contrast, activation differences for nonmatch versus match for congruent Stroop stimuli in older adults showed less frontoparietal network activation than in young adults. Thus, it appears that older adults recruited networks typically involved in conflict resolution but only for response-related cognitive conflict (Madden et al., 1997; Nielson et al., 2002; Lamar et al., 2004), such as when the cue's color did not match the Stroop target's font color but instead matched the incongruent word's content. Only then did older adults engage a frontoparietal cognitive control network more than younger adults, as has been reported elsewhere (Buckner 2005; Cabeza et al., 2002; Grady et al., 2000; Mattay et al., 2006; Reuter-Lorenz, 2002; Rosen et al., 2002). These results provide evidence that older adults are not impaired in the implementation of attentional control strategies, but rather that responsiveness of dorsolateral prefrontal and parietal cortices depends on the type of conflict encountered.
4.4 Gray matter volumes and regional activation patterns in aging
Age-related structural decline may have far-reaching effects in the brain (Kennedy and Raz, 2009) and influence recruitment patterns and cognitive resource availability (Teipel et al., 2007). For example, Voss and colleagues (2008) reported that older adults with more global gray matter volume showed less dedifferentiation, i.e., age-related difficulties in recruiting specialized neural systems. Consistent with other structural imaging studies on aging (Grieve et al., 2005; Raz et al., 1998; Resnick et al., 2003; Schiltz et al., 2006; Sowell et al., 2003) we found smaller gray matter volumes in older than young adults in almost every region of interest obtained by our fMRI analyses. In neither group, however, were regional gray matter volumes significantly related to brain activity in the same region. This demonstrates that older adults were able to modulate gray matter activation according to task demands despite considerable gray matter volume loss. Similarly, it has been reported in MR spectroscopy studies that brain N-acetylasparate (NAA) levels, associated with health and metabolism of local neurons, do not differ in healthy older and young adults when corrected for gray matter volume (Pfefferbaum et al., 1999; Saunders et al., 1999; Zahr et al., 2008). Thus, regional gray matter loss in older adults may not affect functional integrity of the remaining gray matter. It can be speculated that the engagement of different brain networks in older and younger adults is indicative of functional reorganization of neural systems, adaptive to age-related structural decline, to accommodate task demands on perceptual and attentional operations.
One limitation of this study was the relatively small sample size, which may be why we failed to find a relationship between the modulation of regional BOLD responses to task conditions (e.g., incongruency – congruency Stroop contrast) and specific regional gray matter volumes in older and young adults. Additionally, this study did not test for age-related decline in white matter integrity that may also disrupt the connectivity of cortical networks mediating cognitive functions (Schulte et al., 2005; Sullivan et al., 2008; Zahr et al., 2009). However, a recent combined fMRI and DTI study found that age-related compromise of white matter integrity connecting cerebral cortices did not specifically mediate age-related increases in frontoparietal attention network activation (Madden et al., 2007). Future studies that combine fMRI, MRI, and DTI are needed to help understand the role of structural connectivity and cortical integrity in functional network activation for successful conflict resolution in aging.
4.5 Conclusion
Rather than supporting a model of general inhibitory deficits or generally slowed processing by older adults, this study revealed that older adults can effectively use different strategies from the young for perceptual and higher-order cognitive processing by recruiting different brain areas from young adults for inhibitory control. This ability of older adults is consistent with the model CRUNCH, which proposes that processing inefficiencies require the aging brain to recruit more resources to achieve computational output equivalent to that of a younger brain (Reuter-Lorenz and Cappell, 2008). In our Stroop Match-to-Sample task, older adults handled conflict processing by activating a frontoparietal attention system more than young adults and adapted conflict processing to perceptual demands and response repetition by activating a posterior visuomotor network more than young adults. These results support the hypothesis that successful aging is associated with functional reorganization of neural systems to accommodate functionally increasing task demands on perceptual and attentional operations otherwise restricted in normal aging.
Figure 3. Stroop interference and perceptual cueing.
Brain regions demonstrating age differences in Stroop-related activation with matching color cues. The threshold has been lowered to P < 0.005 uncorrected for display purpose. Regions in red reflect greater BOLD response in older than young adults, and regions in green reflect greater BOLD response in young than older adults (left image). Bar graphs are showing mean BOLD signal intensity differences extracted using MarsBaR (http://marsbar.sourceforge.net/) during Stroop-match (INC-CON) performance for older and young adults for regions showing group differences significant at P < 0.001 uncorrected (Table 3).
Acknowledgments
This work was supported by NIA grant AG017919 and NIAAA grants AA010723, AA005965, and AA017168
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement The authors have had no actual or potential financial, personal or other relationships with people or organizations within 3 years of beginning this work that could inappropriately influence or bias their work.
REFERENCES
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bach M. The Freiburg Visual Acuity test--automatic measurement of visual acuity. Optom Vis Sci. 1996;73:49–53. doi: 10.1097/00006324-199601000-00008. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn Affect Behav Neurosci. 2007;7:356–366. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev. 2002;26:809–817. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. June 2–6; 2002. Available on CD-ROM in NeuroImage 16, No 2. [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol Rev. 2007;114:340–375. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Cognitive neuroscience of aging: contributions of functional neuroimaging. Scand J Psychol. 2001;42:277–286. doi: 10.1111/1467-9450.00237. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, Crone EA. Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 2000;97:8728–8733. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cohn NB, Dustman RE, Bradford DC. Age-related decrements in Stroop Color Test performance. J Clin Psychol. 1984;40:1244–1250. doi: 10.1002/1097-4679(198409)40:5<1244::aid-jclp2270400521>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Crovitz HF, Zener K. A group test for assessing hand- and eye-dominance. American Journal of Psychology. 1962;75:271–276. [PubMed] [Google Scholar]
- Davelaar EJ. A computational study of conflict-monitoring at two levels of processing: reaction time distributional analyses and hemodynamic responses. Brain Res. 2008;2:109–119. doi: 10.1016/j.brainres.2007.06.068. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. Neuroimage. 2001;14:1256–1267. doi: 10.1006/nimg.2001.0946. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nat Neurosci. 2005;8:1784–1790. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mentalstate:a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, D'Esposito M. Top-down modulation and normal aging. Ann N Y Acad Sci. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D'Esposito M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci U S A. 2008;105:13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Functional brain imaging and age-related changes in cognition. Biol Psychol. 2000;54:259–281. doi: 10.1016/s0301-0511(00)00059-4. [DOI] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Ann N Y Acad Sci. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. Optimizing the use of information: strategic control of activation of responses. J Exp Psychol Gen. 1992;121:480–506. doi: 10.1037//0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp. 2005;25:391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Shaw M, Yücel M, Purcell R, Brewer WJ, Strother SC, Egan GF, Olver JS, Nathan PJ, Pantelis C. Functional connectivity during Stroop task performance. Neuroimage. 2005;24:181–191. doi: 10.1016/j.neuroimage.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Bunge SA, Scanlon MD, Gabrieli JD. Material-dependent and material-independent selection processes in the frontal and parietal lobes: an event-related fMRI investigation of response competition. Neuropsychologia. 2003;41:1208–1217. doi: 10.1016/s0028-3932(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Humphrey DG, Kramer AF. Age differences in visual search for feature, conjunction, and triple-conjunction targets. Psychol Aging. 1997;12:704–717. doi: 10.1037//0882-7974.12.4.704. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res. 2009;10:41–56. doi: 10.1016/j.brainres.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd., Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Humphrey DG, Larish JF, Logan GD, Strayer DL. Aging and inhibition: beyond a unitary view of inhibitory processing in attention. Psychol Aging. 1994;9:491–512. [PubMed] [Google Scholar]
- Kray J, Eppinger B, Mecklinger A. Age differences in attentional control: an event-related potential approach. Psychophysiology. 2005;42:407–416. doi: 10.1111/j.1469-8986.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Lamar M, Yousem DM, Resnick SM. Age differences in orbitofrontal activation: an fMRI investigation of delayed match and nonmatch to sample. Neuroimage. 2004;21:1368–1376. doi: 10.1016/j.neuroimage.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Nielson KA, Rao SM. fMRI of healthy older adults during Stroop interference. Neuroimage. 2004;21:192–200. doi: 10.1016/j.neuroimage.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Langley LK, Vivas AB, Fuentes LJ, Bagne AG. Differential age effects on attention-based inhibition: inhibitory tagging and inhibition of return. Psychol Aging. 2005;20:356–360. doi: 10.1037/0882-7974.20.2.356. [DOI] [PubMed] [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. J Exp Psychol Hum Percept Perform. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Kaufman DA, Perlstein WM. Neural time course of conflict adaptation effects on the Stroop task. Neuropsychologia. 2009;47:663–670. doi: 10.1016/j.neuropsychologia.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Li S-C, Lindenberger U, Sikström S. Aging cognition: from neuromodulation to representation. Trends in Cognitive Sciences. 2001;5:479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulated cortex in cognitive control. Science. 2000;9:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- McLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Hawk TC, Hoffman JM, Coleman RE. Selective and divided visual attention: age related changes in regional cerebral blood flow measured by H215O PET. Hum Brain Mapp. 1997;5:389–409. doi: 10.1002/(SICI)1097-0193(1997)5:6<389::AID-HBM1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Langley LK. Age-related changes in selective attention and perceptual load during visual search. Psychol Aging. 2003;18:54–67. doi: 10.1037/0882-7974.18.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Cabeza R, Huettel SA. Age-related preservation of top-down attentional guidance during visual search. Psychol Aging. 2004;19:304–309. doi: 10.1037/0882-7974.19.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA. Adult age differences in the functional neuroanatomy of visual attention: a combined fMRI and DTI study. Neurobiol Aging. 2007;28:459–476. doi: 10.1016/j.neurobiolaging.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Coles MG, Hulstijn W, Toni I. Delay-related cerebral activity and motor preparation. Cortex. 2008;44:507–520. doi: 10.1016/j.cortex.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci Lett. 2006;9:32–37. doi: 10.1016/j.neulet.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale (DRS) Professional Manual. Psychological Assessment Resources, Inc.; Odessa, FL: 1988. motor tests in healthy elderly men and women: Exploratory findings. Brain Imag. [Google Scholar]
- Maylor EA, Lavie N. The influence of perceptual load on age differences in selective attention. Psychol Aging. 1998;13:563–573. doi: 10.1037//0882-7974.13.4.563. [DOI] [PubMed] [Google Scholar]
- Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nat Neurosci. 2003;6:450–452. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- Meehan SK, Staines WR. Task-relevance and temporal synchrony between tactile and visual stimuli modulates cortical activity and motor performance during sensory-guided movement. Hum Brain Mapp. 2009;30:484–496. doi: 10.1002/hbm.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher T, Gruber O. Oddball and incongruity effects during Stroop task performance: a comparative fMRI study on selective attention. Brain Res. 2006;1121:136–149. doi: 10.1016/j.brainres.2006.08.120. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain Cogn. 2002;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT. Anterior cingulate cortex: an fMRI analysis of conflict specificity and functional differentiation. Hum Brain Mapp. 2005;25:328–35. doi: 10.1002/hbm.20110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Malamut BL, Bachevalier J. Memories and habits: Two neural systems. In: Lynch G, McGaugh L, Weinberger NM, editors. Neurobiology of learning and memory. Guilford Press; New York: 1984. pp. 65–77. [Google Scholar]
- Müller-Oehring EM, Schulte T, Raassi C, Pfefferbaum A, Sullivan EV. Local-global interference is modulated by age, sex and anterior corpus callosum size. Brain Res. 2007;1142:189–205. doi: 10.1016/j.brainres.2007.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychol Aging. 2004;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cereb Cortex. 2008;18:1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO. In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal aging. Magn Reson Med. 1999;41:276–284. doi: 10.1002/(sici)1522-2594(199902)41:2<276::aid-mrm10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Rabbitt P. An age-decrement in the ability to ignore irrelevant information. J Gerontol. 1965;20:233–238. doi: 10.1093/geronj/20.2.233. [DOI] [PubMed] [Google Scholar]
- Rajah MN, D'Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of aging and cognition. 2nd ed Erlbaum; Mahwah, NJ: 2000. pp. 1–90. [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;15:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Lamar M, Driscoll I. Vulnerability of the orbitofrontal cortex to age-associated structural and functional brain changes. Ann N Y Acad Sci. 2007;1121:562–575. doi: 10.1196/annals.1401.027. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P. New visions of the aging mind and brain. Trends Cogn Sci. 2002;6:394. doi: 10.1016/s1364-6613(02)01957-5. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008;17:177–182. [Google Scholar]
- Rosen AC, Prull MW, O'Hara R, Race EA, Desmond JE, Glover GH, Yesavage JA, Gabrieli JD. Variable effects of aging on frontal lobe contributions to memory. Neuroreport. 2002;20:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Saunders D, Howe F, van den Boogaart A, Griffiths J, Brown M. Aging of the adult human brain: in vivo quantitation of metabolite content with proton magnetic resonance spectroscopy. J Magn Reson Imaging. 1999;9:711–716. doi: 10.1002/(sici)1522-2586(199905)9:5<711::aid-jmri14>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Schiltz K, Szentkuti A, Guderian S, Kaufmann J, Münte TF, Heinze HJ, Düzel E. Relationship between hippocampal structure and memory function in elderly humans. J Cogn Neurosci. 2006;18:990–1003. doi: 10.1162/jocn.2006.18.6.990. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC. MRI Atlas of the Human Cerebellum. Academic Press; San Diego: 2000. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK. The Handbook of Aging and Cognition. Second Edition Lawrence Erlbaum Associates Publishers; 2000. Implications of Perceptual Deterioration for Cognitive Aging Research; pp. 155–219. [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, Maclin EL, Gratton G, Fabiani M. Span, CRUNCH, and Beyond: Working Memory Capacity and the Aging Brain. J Cogn Neurosci. 2009 Mar 25; doi: 10.1162/jocn.2009.21230. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Mueller-Oehring EM, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Differential effect of HIV infection and alcoholism on conflict processing, attentional allocation, and perceptual load: evidence from a Stroop Match-to-Sample task. Biol Psychiatry. 2005;57:67–75. doi: 10.1016/j.biopsych.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Salo R, Pfefferbaum A, Sullivan EV. Callosal involvement in a lateralized stroop task in alcoholic and healthy subjects. Neuropsychology. 2006;20:727–736. doi: 10.1037/0894-4105.20.6.727. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Pfefferbaum A, Sullivan EV. Callosal compromise differentially affects conflict processing and attentional allocation in alcoholism, HIV-infection, and their comorbidity. Brain Imaging and Behavior. 2008;2:27–38. doi: 10.1007/s11682-007-9014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Müller-Oehring EM, Vinco S, Hoeft F, Pfefferbaum A, Sullivan EV. Double dissociation between action-driven and perception-driven conflict resolution invoking anterior versus posterior brain systems. Neuroimage. 2009;48:381–390. doi: 10.1016/j.neuroimage.2009.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldan A, Gazes Y, Hilton HJ, Stern Y. Aging does not affect brain patterns of repetition effects associated with perceptual priming of novel objects. J Cogn Neurosci. 2008;20:1762–1776. doi: 10.1162/jocn.2008.20130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Moeller J, Scarmeas N, Anderson KE, Hilton HJ, Flynn J, Sackeim H, van Heertum R. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex. 2005:394–402. doi: 10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel MP, ten Tusscher MP, Metsemakers JF, Willems B, Jolles J. Visual determinants of reduced performance on the Stroop color-word test in normal aging individuals. J Clin Exp Neuropsychol. 2001;23:620–627. doi: 10.1076/jcen.23.5.620.1245. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;12:643–662. [Google Scholar]
- Teipel SJ, Bokde AL, Born C, Meindl T, Reiser M, Möller HJ, Hampel H. Morphological substrate of face matching in healthy ageing and mild cognitive impairment: a combined MRI-fMRI study. Brain. 2007;130:1745–1758. doi: 10.1093/brain/awm117. [DOI] [PubMed] [Google Scholar]
- Trinkler I, King JA, Doeller CF, Rugg MD, Burgess N. Neural bases of autobiographical support for episodic recollection of faces. Hippocampus. 2009 Jan 27; doi: 10.1002/hipo.20556. in press. [DOI] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Chaddock L, Prakash RS, Colcombe SJ, Morris KS, Doerksen S, Hu L, McAuley E, Kramer AF. Dedifferentiation in the visual cortex: an fMRI investigation of individual differences in older adults. Brain Res. 2008;9:121–131. doi: 10.1016/j.brainres.2008.09.051. [DOI] [PubMed] [Google Scholar]
- Wallentin M, Roepstorff A, Glover R, Burgess N. Parallel memory systems for talking about location and age in precuneus, caudate and Broca's region. Neuroimage. 2006;32:1850–1864. doi: 10.1016/j.neuroimage.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Ward NS. Compensatory mechanisms in the aging motor system. Ageing Res Rev. 2006;5:239–254. doi: 10.1016/j.arr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Pfefferbaum A, Sullivan EV. Low striatal glutamate levels underlie cognitive decline in the elderly: evidence from in vivo molecular spectroscopy. Cereb Cortex. 2008;18:2241–2250. doi: 10.1093/cercor/bhm250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zysset S, Schroeter ML, Neumann J, Yves von Cramon D. Stroop interference, hemodynamic response and aging: An event-related fMRI study. Neurobiol Aging. 2007;28:937–946. doi: 10.1016/j.neurobiolaging.2006.05.008. [DOI] [PubMed] [Google Scholar]