Abstract
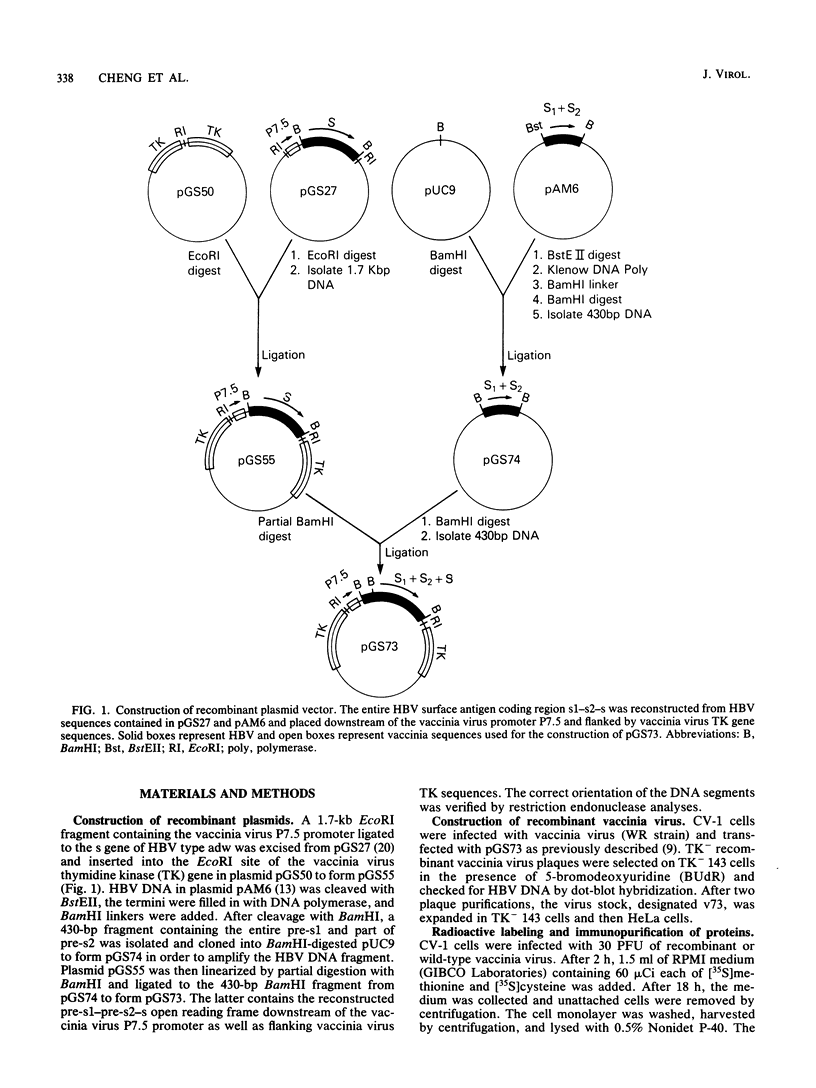
The envelope region of the hepatitis B virus (HBV) genome contains an open reading frame that begins upstream of the major surface protein gene. The two minor proteins that are initiated within this pre-s segment are immunogenic and may be involved in virus attachment to hepatocytes. We have constructed a recombinant vaccinia virus that contains the predicted coding segment for the large surface protein (LS) under control of a vaccinia virus that contains the predicted coding segment for the large surface protein (LS) under control of a vaccinia virus promoter. Cells infected with the recombinant virus synthesized HBV polypeptides of 39 and 42 kilodaltons, corresponding to the unglycosylated and glycosylated forms of LS, respectively. The presence of pre-s epitopes in the 39- and 42-kilodalton polypeptides was demonstrated by binding of antibody prepared against a synthetic peptide. Synthesis of the 42-kilodalton species was specifically inhibited by tunicamycin, suggesting that it is N-glycosylated. Despite apparent glycosylation, LS was not secreted into the medium of infected cells. Nevertheless, rabbits vaccinated with the purified recombinant virus made antibodies that recognized s and pre-s epitopes. Antibody to the NH2 terminus of LS appeared before or simultaneously with antibody that bound to the major surface protein. The additional immunogenicity provided by expression of LS may be advantageous for the development of an HBV vaccine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cattaneo R., Will H., Hernandez N., Schaller H. Signals regulating hepatitis B surface antigen transcription. Nature. 1983 Sep 22;305(5932):336–338. doi: 10.1038/305336a0. [DOI] [PubMed] [Google Scholar]
- Heermann K. H., Goldmann U., Schwartz W., Seyffarth T., Baumgarten H., Gerlich W. H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984 Nov;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laub O., Rall L. B., Truett M., Shaul Y., Standring D. N., Valenzuela P., Rutter W. J. Synthesis of hepatitis B surface antigen in mammalian cells: expression of the entire gene and the coding region. J Virol. 1983 Oct;48(1):271–280. doi: 10.1128/jvi.48.1.271-280.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. A., Green N., Alexander H., Liu F. T., Sutcliffe J. G., Shinnick T. M. Chemically synthesized peptides predicted from the nucleotide sequence of the hepatitis B virus genome elicit antibodies reactive with the native envelope protein of Dane particles. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3403–3407. doi: 10.1073/pnas.78.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida A., Kishimoto S., Ohnuma H., Miyamoto H., Baba K., Oda K., Nakamura T., Miyakawa Y., Mayumi M. A hepatitis B surface antigen polypeptide (P31) with the receptor for polymerized human as well as chimpanzee albumins. Gastroenterology. 1983 Aug;85(2):268–274. [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marglin A., Merrifield R. B. Chemical synthesis of peptides and proteins. Annu Rev Biochem. 1970;39:841–866. doi: 10.1146/annurev.bi.39.070170.004205. [DOI] [PubMed] [Google Scholar]
- Michel M. L., Pontisso P., Sobczak E., Malpièce Y., Streeck R. E., Tiollais P. Synthesis in animal cells of hepatitis B surface antigen particles carrying a receptor for polymerized human serum albumin. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7708–7712. doi: 10.1073/pnas.81.24.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., Thornton G. B., Neurath A. R., Kent S. B., Michel M. L., Tiollais P., Chisari F. V. Enhanced immunogenicity of the pre-S region of hepatitis B surface antigen. Science. 1985 Jun 7;228(4704):1195–1199. doi: 10.1126/science.2408336. [DOI] [PubMed] [Google Scholar]
- Moss B., Smith G. L., Gerin J. L., Purcell R. H. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature. 1984 Sep 6;311(5981):67–69. doi: 10.1038/311067a0. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Strick N., Taylor P., Stevens C. E. Hepatitis B virus contains pre-S gene-encoded domains. Nature. 1985 May 9;315(6015):154–156. doi: 10.1038/315154a0. [DOI] [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R., Samsonoff C., Mercer S., Panicali D. Construction of live vaccines using genetically engineered poxviruses: biological activity of vaccinia virus recombinants expressing the hepatitis B virus surface antigen and the herpes simplex virus glycoprotein D. Proc Natl Acad Sci U S A. 1984 Jan;81(1):193–197. doi: 10.1073/pnas.81.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. A frameshift mutation in the pre-S region of the human hepatitis B virus genome allows production of surface antigen particles but eliminates binding to polymerized albumin. Proc Natl Acad Sci U S A. 1985 May;82(10):3440–3444. doi: 10.1073/pnas.82.10.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcel C., Louise A., Gervais M., Chenciner N., Dubois M. F., Tiollais P. Transcription of the hepatitis B surface antigen gene in mouse cells transformed with cloned viral DNA. J Virol. 1982 Apr;42(1):100–105. doi: 10.1128/jvi.42.1.100-105.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall L. B., Standring D. N., Laub O., Rutter W. J. Transcription of hepatitis B virus by RNA polymerase II. Mol Cell Biol. 1983 Oct;3(10):1766–1773. doi: 10.1128/mcb.3.10.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Mackett M., Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature. 1983 Apr 7;302(5908):490–495. doi: 10.1038/302490a0. [DOI] [PubMed] [Google Scholar]
- Standring D. N., Rutter W. J., Varmus H. E., Ganem D. Transcription of the hepatitis B surface antigen gene in cultured murine cells initiates within the presurface region. J Virol. 1984 May;50(2):563–571. doi: 10.1128/jvi.50.2.563-571.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibbe W., Gerlich W. H. Structural relationships between minor and major proteins of hepatitis B surface antigen. J Virol. 1983 May;46(2):626–628. doi: 10.1128/jvi.46.2.626-628.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]