Abstract
Studies of current interactions among species, their prey, and environmental factors are essential for mitigating immediate threats to population viability, but the true range of behavioral and ecological flexibility can be determined only through research on deeper timescales. Ecological data spanning centuries to millennia provide important contextual information for long-term management strategies, especially for species that now are living in relict populations. Here we use a variety of methods to reconstruct bald eagle diets and local abundance of their potential prey on the Channel Islands from the late Pleistocene to the time when the last breeding pairs disappeared from the islands in the mid-20th century. Faunal and isotopic analysis of bald eagles shows that seabirds were important prey for immature/adult eagles for millennia before the eagles’ local extirpation. In historic times (A.D. 1850–1950), however, isotopic and faunal data show that breeding bald eagles provisioned their chicks with introduced ungulates (e.g., sheep), which were locally present in high densities. Today, bald eagles are the focus of an extensive conservation program designed to restore a stable breeding population to the Channel Islands, but native and nonnative prey sources that were important for bald eagles in the past are either diminished (e.g., seabirds) or have been eradicated (e.g., introduced ungulates). In the absence of sufficient resources, a growing bald eagle population on the Channel Islands could expand its prey base to include carrion from local pinniped colonies, exert predation pressure on a recovering seabird population, and possibly prey on endangered island foxes.
Keywords: Haliaeetus leucocephalus, historic ecology, stable isotopes
Bald eagles (Haliaeetus leucocephalus) were once a familiar apex predator and scavenger in ecosystems on and around California's Channel Islands (CI) (Fig. 1). As a result of direct (e.g., shooting, egg collection, poisoning) and indirect (e.g., pesticides, resource competition) interactions with humans, bald eagles disappeared as a resident breeder on the islands by the mid- to late 1960s (1, 2). Similar to other bald eagle populations in North America, the decline in breeding pairs on the CI coincided with the extensive application of dichlorodiphenyltrichloroethane (DDT) as an agricultural insecticide (1, 3). Although DDT use was banned in the 1970s, bald eagles have been unable to reestablish breeding populations naturally on the CI because of high residual concentrations in local marine food webs (3).
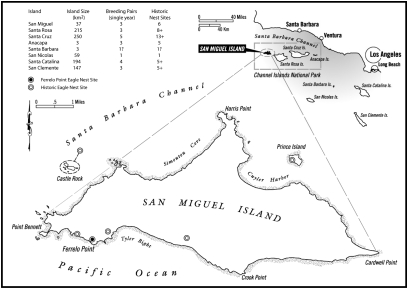
Fig. 1.
Map of SMI showing position relative to other islands in the California CI archipelago. Locations of Ferrelo Point nest (●) and other historic nests known from SMI (○) are shown. Island size (km2) and estimated number of historic bald eagle nest sites and breeding pairs are shown for all islands in the archipelago.
The CI have been the focus of recent conservation efforts aimed at restoring bald eagle populations to historic levels. Initially, reintroduction programs focused on Santa Catalina (SCAT), where translocation and release of juvenile birds began in the early 1980s. Since 1980, 54 juvenile eagles have been released, and 35 chicks have been fostered into active nests on the island (4). The reintroduction program on SCAT has had mixed results, however. The release of juvenile birds has established a small resident population, and eight chicks were hatched in five nests in 2009, but high background DDT and dichlorodiphenyldichloroethylene concentrations in the marine ecosystems that bald eagles depend on has contributed to abnormally high rates of reproductive failure (5).
In 2002, a similar effort was undertaken to establish a breeding population of bald eagles on the northern Channel Islands (NCI). From 2002 to 2006, 61 juvenile eagles were released onto Santa Cruz Island (SCI) (6), resulting in two successful nesting attempts on SCI in 2006 and several unsuccessful nesting attempts on SCI and Santa Rosa Island (SRI) from 2007 to 2009. Although the successful nesting attempts suggest that the ecosystems in the NCI may have sufficiently low contaminant levels to hatch chicks consistently, further reproductive success is required to establish a sustainable breeding population.
Important to the successful reestablishment of bald eagle populations on the NCI and elsewhere in coastal southern California is understanding the types of prey bald eagles consumed in the decades, centuries, and millennia before their local extirpation. Here we use a variety of methods to reconstruct bald eagle diets on San Miguel Island (SMI) in the NCI from the late Pleistocene (∼12–40 ka) to the time when the last breeding pairs disappeared from the islands in the mid-20th century. We focus on SMI because remains of eagles and their prey have been recovered from an historic eagle nest (7) and several paleontological sites (8). We also analyzed historic eagle bones and feathers collected from other CI and the adjacent mainland to characterize historic eagle diets on a regional scale. Because land-use histories are similar throughout the NCI, our results from SMI probably provide a reliable dietary proxy for prehistoric and historic eagle populations on adjacent SRI and SCI (Fig. 1). Our data provide an ecological baseline on trophic dynamics and interaction strengths among eagles and their prey that is integral to understanding how these ecosystems functioned before and during extensive anthropogenic disturbance.
Bald eagles are opportunistic generalists that consume a wide variety of prey via direct capture, scavenging, and/or stealing from other consumers (9). Bald eagles favor freshwater or marine fish, when locally available, over other classes of prey. In the coastal setting of the CI, direct observation and examination of prey remains from nests of reintroduced birds show that, in addition to marine fish, seabirds and waterfowl comprise a significant proportion of their diet (10). Bald eagle nests examined on SCAT from 1991 to 1998 contained on average 86.0% fish, 9.7% birds, and 3.7% mammals, based on the number of prey delivered to nests by adults (10). No published dietary studies are available for the reintroduced eagles on NCI; however, because of the existence of large seabird breeding colonies (11–13), birds probably are more important as prey on the NCI than on SCAT . Faunal analysis of prey remains from an historically occupied (i.e., pre-1960) nest at Ferrelo Point on SMI, for instance, showed that birds comprised a larger proportion of prey than on SCAT (7, 14); with bird remains (52.9%) more common than marine fish (40.7%) as a percentage of the number of identifiable specimens (NISP) in the nest.
Human land use on the NCI has a complex but rich history that dates back millennia. Archaeological evidence shows the islands were first settled by Native Americans in the late Pleistocene (>13 ka), and the Island Chumash harvested a diverse assemblage of marine and terrestrial resources for millennia (15, 16). The historic period began with Spanish colonization of California in the late 1700s, and by about A.D. 1820 Chumash settlements were removed from the CI. Initially, island marine and terrestrial ecosystems may have rebounded after removal of the Chumash, but the replacement of subsistence-based hunting/gathering by commercial harvest of sea otters (Enhydra lutris), pinnipeds, abalone, and fish for the global fur, oil, and food markets resulted in unprecedented ecological changes in marine communities (16). By the 1850s, sheep (Ovis aries) and other domesticated ungulates [cattle (Bos taurus), horses (Equus ferus), goats (Capra aegagrus), and pigs (Sus scrofa)] were introduced to the islands, initiating a century of ranching (17) and continued detrimental changes in the islands’ terrestrial ecosystems. Historic commercial records suggest that all of the NCI were severely overgrazed, resulting in vegetation loss, soil erosion, and destabilization of island dune systems (18), as well as destruction of seabird breeding habitats (11). Sheep ranching continued on one or more of the NCI until the mid-20th century (Fig. 2A), when programs were initiated to eradicate nonnative ungulates (19–22). Except for mule deer (Odocoileus hemionus) and elk (Cervus canadensis) on SRI, the last ungulates (feral pigs) in the NCI were removed from SCI in 2006 (22).
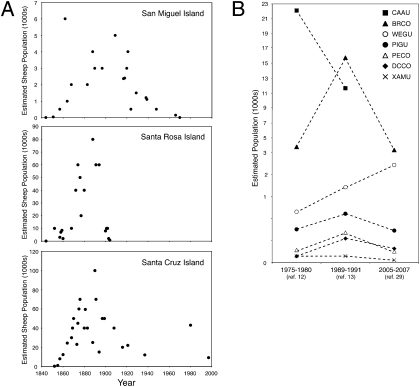
Fig. 2.
Historic trends in the population sizes of feral sheep (A) and seabirds (B) on the northern Channel Islands. (A) Historic estimates of sheep population sizes on the three largest NCI (SMI, SRI, and SCI) throughout the historic period. (B) Historic estimates of seabird population sizes. BRCO, Brandt's cormorant; CAAU. Cassin's auklet; DCCO, double-crested cormorant; PECO, pelagic cormorant; PIGU, pigeon guillemot (Cepphus columba); WEGU, Western gull (Larus occidentalis); XAMU, Xantus's murelet.
Overall, the historic human presence on the NCI has led to an alteration of (i) marine community dynamics because of significant decreases in the diversity and abundance of key species (e.g., sea otter, abalone, fish); and (ii) terrestrial ecosystems via the introduction of nonnative ungulates. For bald eagles, this complex human land-use history probably resulted in a decrease in the availability of marine resources but concomitant increases in nonnative terrestrial prey. Given the intensive efforts of the bald eagle reintroduction program on the CI in recent years (4, 6), it is important to understand how temporal changes in the state of the island's marine and terrestrial ecosystems influenced resident bald eagle populations. This appreciation in turn requires an understanding of energy flow among eagles and their environment at different time periods, an understanding that is essential for determining how recent changes in environmental conditions—natural or otherwise—have shaped their ecology.
Stable isotope analysis (SIA) of bone collagen and feather keratin archived in paleontological, archaeological, and historical museum collections is an established method for characterizing shifts in the foraging ecology of animals through time (23). SIA is especially useful for differentiating marine from terrestrial resource use because of baseline differences in the isotopic composition of primary producers in marine versus terrestrial ecosystems. In California, coastal marine ecosystems are characterized by higher δ13C and δ15N values than their adjacent terrestrial counterparts. In coastal California, terrestrial primary productivity is dominated by C3 photosynthesis (24), resulting in food webs characterized by relatively low δ13C values ranging from −22 to −28‰ (25). Coastal marine ecosystems, in contrast, are dominated by a combination of micro- and macroalgae that have higher δ13C values (−16 to −20‰) (26). For nitrogen, field and laboratory-based studies have established that there is an increase in δ15N values of ∼3–5‰ per trophic step in marine and terrestrial ecosystems (27). Because coastal marine systems contain a greater number of trophic levels than their terrestrial counterparts, they typically have higher δ15N values; apex predators in marine and terrestrial ecosystems have δ15N values in the range of +16–19‰ and +7–12‰, respectively. Consumers that rely on a mixture of marine and terrestrial resources have intermediate δ15N values ranging from +12–16‰. Although δ13C and δ15N values within terrestrial or marine systems can change by ∼1–3‰ spatially and temporally in response to a wide variety of physical (e.g., climatic or oceanographic) and biological (e.g., phytoplankton growth rate) factors, the large 6–10‰ gradient in both carbon and nitrogen isotope values among secondary and tertiary consumers in marine and terrestrial ecosystems provides a robust proxy for examining marine versus terrestrial resource use in a top predator that utilizes both ecosystems. Likewise, anthropogenic changes in the δ13C baseline of terrestrial and marine ecosystems caused by the burning of fossil fuels (i.e., Suess effect) is relatively minor (∼0.5‰) when examining historic specimens that date from the early to mid-20th century.
Results
Ferrelo Point Nest Prey Abundance.
The Ferrelo Point nest is located on the southwest coast of SMI (Fig. 1) and was perched on the rim of a steep escarpment, atop a rock outcrop ∼99 m above sea level (7, 14). A 1939 photograph shows a bald eagle chick on the Ferrelo Point nest, a large structure ∼2.8 m tall and ∼2 m wide that probably was used by eagles for more than 100 years. Excavation of the nest produced nearly 10,000 faunal elements, 98% of them from vertebrates (birds, fish, reptiles, and mammals) and 2% from mollusks and other invertebrates (7, 14). NISP and minimum number of individuals (MNI) metrics suggest that seabirds, followed by marine fishes and mammals, were the most important dietary taxa for bald eagles at Ferrelo Point (Tables S1 and S2) (7).
Isotopic Analysis of Bald Eagles and Potential Prey.
Isotopic analysis of prey found in the Ferrelo Point nest (Fig. 3) presents a pattern expected of a terrestrial-to-marine gradient in California. Terrestrial prey species [sheep and island foxes (Urocyon littoralis)] have significantly lower mean δ13C and δ15N values (t test, P < 0.05) than marine species (seabirds and fish). Bird species that use both marine and freshwater habitats throughout their annual life cycle [e.g., eared grebes (Podiceps nigricollis) and scoters (Melanitta spp.)] have intermediate mean δ13C and δ15N values. Within the marine realm, surfperch (Embiotocidae) and rockfish (Sebastes spp.) have significantly higher mean δ13C values and lower δ15N values than most seabird species analyzed (ANOVA, P < 0.05).
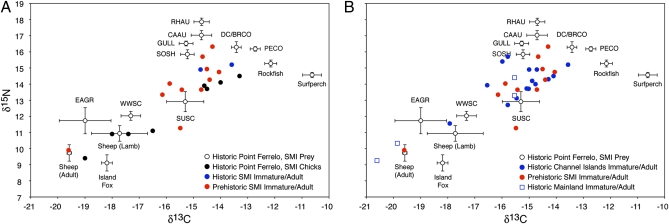
Fig. 3.
Bivariate plot of mean δ13C for δ15N values for bald eagles and the most abundant prey items found in the Ferrelo Point nest on SMI. (A) Bald eagle specimens collected on SMI from historic and prehistoric contexts. (B) Historic and prehistoric bald eagle specimens collected from throughout the CI and adjacent mainland. Error bars represent SE. Table S1 presents sample sizes of potential prey. CAAU, Cassin's auklet; DC/BRCO, double-crested or Brandt's cormorant; EAGR, eared grebe; GULL, large gulls (Larus spp.); PECO, pelagic cormorant; RHAU, rhinoceros auklet; SOSH, sooty shearwater (Puffinus griseus); SUSC, surf scoter (Melanitta perspicillata); WWSC, white-winged scoter (Melanitta fusca). Sheep were grouped into separate age classes (lambs and adults) because of significant differences in mean δ13C values (t test, P < 0.05). Bald eagle bone collagen isotope values were corrected for trophic discrimination by subtracting 1.5‰ and 3.0‰, respectively, from measured δ13C for δ15N values. Bald eagle feather isotope values were corrected for tissue-specific and trophic discrimination by adding 1.5‰ and subtracting 3.0‰ from measured δ13C for δ15N values. Further clarification on discrimination factors is given in Materials and Methods and SI Text. Table S3 gives the collection location, collection year, and age of each bald eagle specimen.
To make direct isotopic comparisons among bald eagles and their potential prey (Fig. 3), we corrected eagle bone and feather values for trophic and tissue-specific discrimination (SI Text). Our isotopic comparisons (Fig. 3) are presented in bone collagen isotopic space, so all eagle bone collagen values were corrected only for trophic discrimination by subtracting 1.5‰ and 3‰ from measured δ13C and δ15N values, respectively (23). Correction of historic bald eagle feathers required the application of both a tissue-specific and trophic discrimination factor. To correct feathers for both trophic and tissue-specific discrimination, we added ∼1.5‰ to eagle keratin δ13C values (Fig. 3). Tissue-specific corrections are not required for δ15N, but feathers must be corrected for trophic discrimination by subtracting 3‰. These discrimination factors are estimates, and recent studies show that they can vary at the taxonomic and individual level (27). For the large marine versus terrestrial isotopic gradients used here, however, relatively small inter-individual variations in discrimination factors do not compromise our assessment of bald eagle diets through time.
Isotopic analysis of historic and prehistoric bald eagles from SMI (Fig. 3A) suggests that eagles used both marine and terrestrial prey. Half (four of eight) of the historic bald eagle chicks from Ferrelo Point plot solidly within the terrestrial prey space (solid black circles, Fig. 3A), suggesting a diet primarily composed of sheep, which is supported by NISP and MNI metrics of terrestrial prey remains identified in the nest (Table S1). The other four historic chicks have δ13C values suggestive of a marine diet, but their trophic corrected δ15N values are significantly lower than those of most marine prey found in the nest (Fig. 3A), suggesting that they also consumed some terrestrial or mixed freshwater/marine prey (e.g., eared grebes and scoters). Two immature/adult historic eagles from SMI had δ13C and δ15N values suggestive of a marine diet consisting primarily of seabirds (solid blue circles, Fig. 3A). The prehistoric eagles from SMI also show a wide range in δ13C and δ15N values (solid red circles, Fig. 3A). Most prehistoric individuals have isotope values indicative of a principally marine or mixed marine/terrestrial diet, but one individual (VP 43 #1, Table S3) has much lower δ13C and δ15N values indicative of a terrestrial-based diet.
Isotopic analysis of historic bald eagle bones and feathers from other CI and the adjacent mainland (solid blue circles, Fig. 3B) also shows a mixed pattern of marine and terrestrial prey use. Most individuals from the CI have isotope values indicative of a marine prey base, but several individuals have δ15N values indicating a mixed marine/terrestrial diet, and one individual plots solidly within the terrestrial realm. Two historic individuals collected from coastal areas on the mainland have values indicative of a marine prey base (open blue squares Fig. 3B); the other two collected from inland mainland localities have relatively low isotope values indicative of a terrestrial diet.
Historic Sheep, Seabird, and Marine Mammal Population Estimates.
Sheep were first introduced onto the NCI in the mid- to late 1840s (SRI) and early 1850s (SMI and SCI). The number of sheep on each of the islands grew significantly through the latter half of the 19th century (Fig. 2A) and then declined during the first half of the 20th century when ranching operations were transitioning from sheep to cattle grazing on the larger NCI (SRI and SCI). During the first half of the 20th century, 1,100–2,500 sheep were on SMI. After the termination of the grazing lease in 1948, all but 500 sheep were removed from the island immediately. The last sheep on SMI were eliminated in 1966, and today no feral herbivores occur on the island. Sheep grazing declined on SRI in the early 1900s, the number of sheep decreased from ∼10,000 to ∼700 between 1901 and 1904, and the last sheep were removed in the early 1950s. Despite the transition from a sheep- to a cattle-grazing operation in the early 1900s, a significant number of feral sheep remained on SCI until near the end of the 20th century, when sheep and other feral ungulates (e.g., pigs) finally were eradicated from the island (19, 20).
Until the late 1970s, there were no accurate island-specific standardized population counts for seabirds nesting on the CI. Systematic surveys undertaken in the late 1970s (11, 12), late 1980s (13), and between the mid-1990s and 2007 (28) provide estimates for more recent seabird populations (Fig. 2B). By the late 1980s the seabird population on SMI and adjacent islets was estimated to be ∼33,250 birds of at least 12 species (13). By 2007 this avifauna was estimated to be ∼20,780 birds of 13 species (28). Population estimates for most species have not varied greatly between each of these survey efforts (Fig. 2B). Double-crested cormorants (Phalacrocorax auritus) declined between the late 1970s and 1980s, Brandt's cormorants (Phalacrocorax penicillatus) increased during the late 1980s but returned to late 1970s numbers by 2005–2007, and Western gulls (Larus occidentalis) increased between the late 1970s and 2007 (Fig. 2B).
Archaeological data show that pinnipeds and sea otters were present throughout the Holocene, although the relative abundance of species fluctuated through time (16, 29). During the 19th century, however, the rapid growth of a global fur and oil industry focused on commercial hunting led to the extirpation of sea otters and severe declines in pinniped populations in southern California (30–33). Under federal protection these populations have rebounded in recent decades (33, 34), but they may have been reduced dramatically or were absent from NCI ecosystems during the period that the Point Ferrelo nest was used by bald eagles.
Discussion
Isotopic analysis of the most abundant prey found in the historic Ferrelo Point nest on SMI presents a pattern expected of a marine-to-terrestrial gradient in southern California (Fig. 3). Marine species (e.g., fish and seabirds) have higher mean δ13C and δ15N values than their terrestrial counterparts (e.g., sheep and foxes), and species dependent on a seasonal mix of marine and terrestrial food webs (e.g., scoters and eared grebes) have intermediate values. This isotopic gradient allows the identification of marine, terrestrial, or mixed resource use for bald eagles and has been used for decades in paleoecological, archaeological, and ecological studies (24). The general isotopic pattern among marine versus terrestrial prey on SMI also can be used as a dietary proxy for other CI and the adjacent mainland, because these patterns are driven by functional differences between marine and terrestrial ecosystems in the region.
The isotopic composition of bald eagle chicks differs substantially from the overall prey abundance in the historic Ferrelo Point nest (Tables S1 and S2). Birds and fish dominate the prey remains in the nest, together accounting for ∼80% of MNI and ∼90% of NISP, whereas terrestrial mammals represent only ∼5% of MNI or NISP. This pattern contrasts sharply with the isotopic composition of bald eagle chicks from the nest (Fig. 3A). Half (four of eight) of the historic eagle chicks had δ13C and δ15N values indicative of a reliance on terrestrial prey. Because sheep were the most abundant terrestrial prey species found in the nest (∼44% of MNI and ∼77% of NISP of terrestrial taxa) (Table S1), they probably were an important source of prey for historic bald eagle chicks and their parents at Ferrelo Point.
The variable isotopic pattern among bald eagle chicks from Ferrelo Point suggest a diverse prey base in which carrion from domesticated ungulates could have provided an important resource for the resident breeding eagle population. Similar to other temperate latitude breeding pairs, recently reintroduced bald eagles on SCI typically lay eggs in late February to early March with chicks hatching in early to mid-April. This seasonal reproductive schedule coincides with the historic lambing season on the NCI. Lambs were born in January and February and typically comprised ∼30% of the total sheep population through May (35). At the peak of ranching on SMI in the early 1900s, the sheep population exceeded 5,000 individuals (Fig. 2A). Given the relatively small size of SMI (37 km2) and the maximum number of three eagle breeding pairs known to nest on the island in a single year during the historic period (Fig. 1), sheep carcasses could have been a significant source of prey for eagles and their offspring, especially if nearly one third of the sheep population was comprised of lambs, which have lower survival rates than adults. This scenario probably was true for the other NCI as well (Figs. 1 and 2A). Land-use histories on SMI and Anacapa Island (ACI) present a particularly interesting scenario. Today, these islands are major breeding sites for 16 species of seabirds (11), but historic documents show that at the height of ranching in the early 20th century, the islands were home to an extraordinary number of sheep given their relatively small size. Intensive disturbance from sheep ranching probably was a major contributor to the decline of breeding seabird populations in the area (11, 28) but also provided a significant source of terrestrial carrion for bald eagles.
Approximately half of the historic immature/adult eagles collected from across the CI have trophic-corrected δ15N values ≥14‰ and δ13C values in the −14‰ to −16‰ range (solid blue circles, Fig. 3B). These individuals plot closer to mean isotope values for seabirds than for fish, suggesting a heavy reliance on seabird prey. The other historic eagles collected archipelago-wide have intermediate δ13C and δ15N values indicative of mixed marine and terrestrial resource use. These eagles (i) may have consumed a mixed diet of seabirds and avian prey—scoters and eared grebes—that rely on both marine and terrestrial ecosystems at different stages in their annual life cycle; (ii) may have consumed a mixed diet of seabirds and introduced ungulates; or (iii) may have been seasonal migrants from the adjacent mainland. Satellite tracking of reintroduced bald eagles recently released on SCI shows that a small proportion of individuals (4/33; 12%) make seasonal migrations as far north as central British Columbia, often using inland corridors (6). Two other historic immature/adult eagles (open blue circles, Fig. 3B) have isotopic signatures indicative of complete reliance on terrestrial resources, but they were collected on the mainland in inland San Diego County and Orange County (Table S3).
Isotopic data for prehistoric eagles (solid red circles, Fig. 3) collected from paleontological sites on SMI also suggest a heavy reliance on seabird prey, but, as in the data for historic island eagles, several prehistoric individuals have isotopic signatures suggestive of a mixed marine and terrestrial resource base. Two individuals have isotope values that suggest heavy reliance on terrestrial prey (Fig. 3). This result is unexpected, because before Spanish colonization terrestrial CI ecosystems did not contain a diverse assemblage of large (>1 kg) native prey species suitable for bald eagles. Both full-sized Columbian (Mammuthus columbi) and pygmy (M. exilis) mammoths were the only two species of Pleistocene terrestrial megafauna that existed on the NCI, but both perished in the Pleistocene extinction at ∼13 ka (36). The endemic island fox (∼2 kg) and island spotted skunk (Spilogale gracilis, <1 kg), were the largest terrestrial mammals before the historic introduction of nonnative ungulates. Because the stratigraphic provenience of the two terrestrial-dependent eagles securely dates them to the late Pleistocene (8), when terrestrial prey sources were not locally abundant, these individuals probably were migrants from the mainland.
In contrast to the terrestrial scenario, the NCI were home to a diverse community of Pleistocene marine avifauna that our isotope data show were important prey for bald eagles. More than 61 species of seabirds have been found in paleontological sites on SMI (8). Only three of these species—Chendytes lawi, Fratercula dowi, Morus reyanus—are now extinct, but many extant species have suffered historic population declines because of interactions with humans (e.g., hunting, habitat loss). Many seabird species have been protected since the early 1970s, but population estimates compiled since then show a mixed pattern of recovery, stabilization, and even decline (Fig. 2B). Of the seven species for which there are reliable estimates, the two that are currently in significant decline [Cassin's auklet (Ptychoramphus aleuticus) and Brandt's cormorant) are relatively abundant in the historic Ferrelo Point bald eagle nest (Table S1). Cassin's auklet once was the second most abundant seabird breeding in California, but the breeding population on the CI and elsewhere in California declined by as much as 80% from 1985 to 1994 (37). Many other seabird populations are more stable, albeit at low numbers, including Xantus's murrelet (Synthliboramphus hypoleucus) and double-crested and pelagic (P. pelagicus) cormorants. Other species for which there are incomplete records but which are believed to be at lower population sizes in comparison with historic levels, include tufted puffins (Fratercula cirrhata), common murres (Uria aalge), brown pelicans (Pelecanus occidentalis), rhinoceros auklets (Cerorhinca monocerata), and two species of storm petrel (Oceanodroma leucorhoa and O. melania) (11, 28). Many of these seabirds were important prey for the bald eagles nesting at Ferrelo Point on SMI in historic times (Table S1) and are assumed to have been important prey for other resident breeding eagles on the NCI in the past (Fig. 3). Satellite tracking of young, nonterritorial bald eagles recently released on SCI show that many individuals frequent ACI during the spring months when seabirds are present there in large numbers to breed (6).
Marine and terrestrial ecosystems across the CI and southern California have suffered historic shifts in community structure and function, many of which occurred as a direct or indirect result of human activities (33, 38–44). Our data on past resource use for bald eagles present an example in which a native top predator was able to exploit significant and sometimes rapid shifts in the structure of terrestrial and marine ecosystems. Termination of grazing operations on the NCI occurred at approximately the same time that bald eagles disappeared from the NCI, likely because of the effects of DDT exposure. Following the disappearance of bald eagles from CI ecosystems, costly and time-intensive eradication programs were implemented to remediate the effects of ranching on the NCI (19–22). Furthermore, the anthropogenic increase in terrestrial prey for bald eagles in the form of introduced ungulates coincided with a period when native marine prey sources (seabirds, fish, and marine mammals) were in decline across southern California (11, 28, 33, 38–44). Bald eagles are now the focus of an extensive conservation program designed to restore a stable breeding population to the NCI (6), but native and nonnative prey sources are either diminished in comparison with past numbers (e.g., seabirds; Fig. 2B), do not exist (e.g., introduced ungulates; Fig. 2A), or are present in greater abundance today than in the past ∼10 ka (e.g., pinnipeds; refs. 33, 44).
Recent golden eagle (Aquila chrysaetos) predation of island foxes on the NCI resulted in swift and costly action to capture both predator and prey to avoid probable extinction of the fox (40, 45–47). It is hypothesized that golden eagles naturally colonized the NCI to take advantage of the high abundance of feral ungulates and switched to preying on foxes after eradication programs reduced ungulate densities (40, 45, 48). Both golden eagles and feral ungulates now have been removed from the islands, but a monitoring program recently has discovered evidence of island fox predation by eagles on SCI and SRI that occurred after golden eagle removal (49). It is unclear whether these recent predation events were initiated by bald eagles or by transient golden eagles, which are sighted occasionally on the NCI. Because the bald eagle population on the NCI continues to increase as a result of in situ breeding success, it is possible that the endangered island fox could provide a natural terrestrial alternative to the compromised marine prey base available to bald eagles.
Our deep temporal perspective of interactions among bald eagles and their prey on the CI provides conservation and management programs with unique ecological data that span millennia. Our data on temporal changes in resource use can inform wildlife managers about (i) the possible conflicts that a reestablished bald eagle population might have with recovering island fox and seabird populations on the islands; (ii) the prey that eagles are likely to focus on and impact as they reestablish breeding populations on the CI; and (iii) the impacts that contaminated prey (e.g., seabirds and marine mammals) might have on the reproductive success of a recovering bald eagle population. Isotopic data from historic and prehistoric bald eagle individuals throughout the CI suggest that seabirds were an important source of prey in the past but that historic breeding eagles on SMI took advantage of locally abundant introduced ungulates to raise their chicks. With the removal of introduced ungulates from the islands and the recent reductions in native marine resources (e.g., seabirds and fish), a recovering population of bald eagles on the NCI either will emigrate or expand its prey base to include other locally available resources. Because bald eagles are opportunistic foragers known to consume a wide variety of prey in proportion to local availability, a growing eagle population on the NCI is expected to expand its prey base to include carrion from locally abundant pinniped (e.g., Zalophus californianus, Mirounga angustirostris, Callorhinus ursinus) colonies and possibly to prey on endangered island foxes. To restore a sustainable bald eagle population to this region, managers should consider (i) the presently reduced abundance of natural marine prey (e.g., seabirds and fish) in comparison with the past; (ii) the effects on eagles of eliminating introduced ungulates that were an important resource for breeding eagles during the historic period; (iii) the possible adverse effects on eagles from an increased reliance on contaminant-laden carcasses of an abundant pinniped population that breeds on the NCI (50); and (iv) the effects that a growing eagle population will have on recovering seabird colonies and endangered island fox populations on the NCI.
Materials and Methods
Ferrelo Point Nest Prey Abundance.
We identified all of the faunal materials to the lowest possible taxon (SI Text). We quantified the faunal remains in three ways: (i) by bone weight to the nearest 0.1 g; (ii) by the NISP, calculated by counting the total number of elements identified to each taxon; and (iii) by MNI, determined by the largest number of unique elements identified per taxon.
Statistical and Isotopic Analysis of Bald Eagles and Potential Prey.
Isotopic differences among bald eagles prey were assessed using a one-way ANOVA and a post hoc Tukey Honestly Significant Difference test or a nonparametric t test.To isolate collagen, a small bone fragment was demineralized in 0.5 M HCl for ∼36 h at ∼5 °C. Bone collagen and feather samples were treated with a 2:1 chloroform:methanol mixture to remove lipids and surface contaminants and then were lyophilized. Feather isotope values presented in Fig. 3 represent mean values for five subsamples cut from each specimen; within-feather SD was less than 0.5‰. Dried collagen and keratin samples (∼0.5 mg) were sealed in tin capsules and analyzed using a Carlo Erba NC2500 elemental analyzer interfaced with a Thermo-Finigan Delta V mass spectrometer at the Carnegie Institution of Washington (Washington, DC). The C/N ratios were 2.8–3.2 of all collagen samples and were 3.2–3.6 for all keratin samples (Table S3). Information on isotopic notation and laboratory standards is given in SI Text: Isotopic Nomenclature.
Historic Sheep, Seabird, and Marine Mammal Population Estimates.
Historic estimates of sheep, seabird, and marine mammal populations on each of the NCI were compiled from a variety of sources. (More details are given in SI Text: Historic Sheep, Seabird, and Marine Mammal Population Estimates.)
Supplementary Material
Acknowledgments
We thank collections managers and curators at the Academy of Natural Sciences, Philadelphia; the Field Museum of Natural History, Chicago; the Museum of Comparative Zoology, Harvard University, Cambridge; the Museum of Vertebrate Zoology, University of California, Berkeley; the Natural History Museum of Los Angeles County, Los Angeles; the San Diego Natural History Museum, San Diego; the Santa Barbara Museum of Natural History; the Slater Museum of Natural History, University of Puget Sound, Tacoma; the University of California, Los Angeles; the Ventura County Museum of History and Art, Ventura; and the Western Foundation of Vertebrate Zoology, Camarillo for providing historic eagle feather and bone samples. We thank E. A. Swarth, B. J. O'Connor, and N. M. Smith-Herman for laboratory assistance and A. C. Jakle for constructive reviews. The National Park Service provided funding that supported collection and identification of prey remains from the Ferrelo Point bald eagle nest. S.D.N. and M.L.F. was funded in part by National Science Foundation Grant ATM-0502491, by the Carnegie Institution of Washington, and by Grant 07100 from the W.M. Keck Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0913011107/-/DCSupplemental.
References
- 1.Kiff LF. In: The California Islands: Proceedings of a Multidisciplinary Symposium. Power DM, editor. Santa Barbara, CA: Santa Barbara Museum of Natural History; 1980. pp. 651–671. [Google Scholar]
- 2.Kiff LF. Further Notes on Historical Bald Eagle and Peregrine Falcon Populations on the California Channel Islands. Sacramento, CA: US Fish and Wildlife Service; 2000. [Google Scholar]
- 3.Garcelon DK, Roemer GR. In: Endangered Wildlife and Habitats in Southern California. Memoirs of the Natural History Foundation of Orange County No. 3. Bryant PJ, Remington J, editors. Newport, CA: Natural History Foundation of Orange County; 1990. pp. 63–68. [Google Scholar]
- 4.Sharpe PB, Garcelon DK. In: Proceedings of the Sixth California Islands Symposium. Garcelon DK, Schwemm CA, editors. Arcata, CA: Institute for Wildlife Studies; 2005. pp. 323–330. [Google Scholar]
- 5.Garcelon DK, Thomas NJ. DDE poisoning in an adult bald eagle. J Wildl Dis. 1997;33:299–303. doi: 10.7589/0090-3558-33.2.299. [DOI] [PubMed] [Google Scholar]
- 6.Sharpe PB. Bald Eagle Restoration on the Northern Channel Islands, California. Arcata, CA: Institute for Wildlife Studies; 2007. [Google Scholar]
- 7.Collins PW, Guthrie DA, Rick TC, Erlandson JM. In: Proceedings of the Sixth California Islands Symposium. Garcelon DK, Schwemm CA, editors. Arcata, CA: Institute for Wildlife Studies; 2005. pp. 103–120. [Google Scholar]
- 8.Guthrie DA. In: Proceedings of the Sixth California Islands Symposium. Garcelon DK, Schwemm CA, editors. Arcata, CA: Institute for Wildlife Studies; 2005. pp. 35–42. [Google Scholar]
- 9.Stalmaster MV. The Bald Eagle. New York: Universe Books; 1987. [Google Scholar]
- 10.Sharpe PB, Garcelon DK. Analysis of the Potential Diet of Bald Eagles Reintroduced to Santa Cruz and Anacapa Islands, California. Sacramento, CA: US Fish and Wildlife Service; 1999. [Google Scholar]
- 11.Hunt GL, Jr, Pitman RL, Jones HL. In: The California Islands: Proceedings of a Multidisciplinary Symposium. Power DM, editor. Santa Barbara, CA: Santa Barbara Museum of Natural History; 1980. pp. 443–459. [Google Scholar]
- 12.Sowls AL, DeGange AR, Nelson JW, Lester GS. Catalog of California Seabird Colonies. Washington, DC: US Department of the Interior; 1980. FWS/OBS 37/80. [Google Scholar]
- 13.Carter HR, et al. Breeding Populations of Seabirds in California, 1989-1991. Newark, NJ: U. S. Fish and Wildlife Service; 1992. Vol. 1: Population Estimates. [Google Scholar]
- 14.Erlandson JM, Rick TC, Collins PW, Guthrie DA. Archaeological implications of a bald eagle nesting site at Ferrelo Point, San Miguel Island, California. Journal of Archeological Science. 2007;34:255–271. [Google Scholar]
- 15.Rick TC, Erlandson JM, Vellanoweth R, Braje TJ. From Pleistocene mariners to complex hunter-gatherers: The archaeology of the California Channel Islands. J World Prehist. 2005;19:169–228. [Google Scholar]
- 16.Erlandson JM, Rick TC. Archaeology meets marine ecology: The antiquity of maritime cultures and human impacts on marine fisheries and ecosystems. Annu Rev Mar Sci. 2010;2:165–185. doi: 10.1146/annurev.marine.010908.163749. [DOI] [PubMed] [Google Scholar]
- 17.Livingston DS. Ranches in the Sea. A History of the Islands Within Channel Islands National Park. Ventura, CA: National Park Service; 2006. [Google Scholar]
- 18.Johnson DL. In: The California Islands: Proceedings of a Multidisciplinary Symposium. Power DM, editor. Santa Barbara, CA: Santa Barbara Museum of Natural History; 1980. pp. 103–121. [Google Scholar]
- 19.Van Vuren D. Fifteenth Vertebrate Pest Conference. Davis, CA: Univ of California; 1992. pp. 377–381. [Google Scholar]
- 20.Schuyler P. In: Third California Islands Symposium. Hochberg FG, editor. Santa Barbara, CA: Santa Barbara Museum of Natural History; 1993. pp. 443–452. [Google Scholar]
- 21.Lombardo CA, Faulkner KR. In: Proceedings of the Fifth California Islands Symposium. Browne DH, Chaney H, Mitchell K, editors. Santa Barbara, CA: Santa Barbara Museum of Natural History; 2003. pp. 300–306. [Google Scholar]
- 22.Morrison SA. In: Managing Vertebrate Invasive Species: Proceedings of an International Symposium. Witmer GW, Pitt WC, Fagerston KA, editors. Fort Collins, CO: National Wildlife Research Center; 2007. pp. 398–409. [Google Scholar]
- 23.Koch PL. In: Stable Isotopes in Ecology and Environmental Science. Michener R, Lajtha K, editors. Boston: Blackwell Publishing; 2007. pp. 99–154. [Google Scholar]
- 24.Suits NS, et al. Simulation of carbon isotope discrimination of the terrestrial biosphere. Global Biogeochem Cycles. 2005;19:GB1017. [Google Scholar]
- 25.Craig H. The geochemistry of the stable carbon isotopes. Geochim Cosmochim Acta. 1953;3:53–92. [Google Scholar]
- 26.Page HM, Reed DC, Brzezinski MA, Melack JM, Dugan JE. Assessing the importance of land and marine sources of organic matter to kelp forest food webs. Mar Ecol Prog Ser. 2008;360:47–62. [Google Scholar]
- 27.Vanderlift MA, Ponsard S. Sources of variation in consumer-diet δ15N enrichment: A meta-analysis. Oecologia. 2003;136:169–182. doi: 10.1007/s00442-003-1270-z. [DOI] [PubMed] [Google Scholar]
- 28.Carter HR, et al. Status of Breeding Seabirds in the San Miguel Island Group, California. Davis, CA: Victoria and California Institute of Environmental Studies; 2008. [Google Scholar]
- 29.Jones TL, Hildebrandt WR, Kennett DJ, Porcasi JF. Prehistoric marine mammal overkill in the northeastern Pacific: A review of new evidence. Journal of California and Great Basin Anthropology. 2004;24:69–80. [Google Scholar]
- 30.Scammon CM. The Marine Mammals of the Northwest Coast of North America. New York: Dover Publishing Company; 1874. [Google Scholar]
- 31.Ogden A. Russian sea otter and seal hunting on the California coast. Calif Hist Soc Q. 1933;12:29–51. [Google Scholar]
- 32.Cass VL. Exploitation of California sea lions, Zalophus californianus, prior to 1972. Mar Fish Rev. 1985;47:36–38. [Google Scholar]
- 33.Stewart BS, Yochem PK, DeLong RL, Antonelis GA. In: Third California Islands Symposium. Hochberg FG, editor. Santa Barbara, CA: Santa Barbara Museum of Natural History; 1993. pp. 501–516. [Google Scholar]
- 34.Lowry MS, Maravilla-Chavez O. In: Proceedings of the Sixth California Islands Symposium. Garcelon DK, Schwemm CA, editors. Arcata, CA: Institute for Wildlife Studies; 2005. pp. 485–489. [Google Scholar]
- 35.Van Vuren D, Coblentz BE. Population characteristics of feral sheep on Santa Cruz Island. J Wildl Manage. 1989; 53:306–313. [Google Scholar]
- 36.Agenbroad LD, Johnson JR, Morris D, Stafford TW., Jr . In: Proceedings of the Sixth California Islands Symposium. Garcelon DK, Schwemm CA, editors. Arcata, CA: Institute for Wildlife Studies; 2005. pp. 3–7. [Google Scholar]
- 37.Ainley DG, Sydeman WJ, Hatch S, Wilson U. Seabird population trends along the west coast of North America: Causes and the extent of regional concordance. Studies in Avian Biology. 1994;15:119–133. [Google Scholar]
- 38.Dayton PK, Tegner MJ, Edwards PB, Riser KL. Sliding baselines, ghosts, and reduced expectations in kelp forest communities. Ecol Apps. 1998;8:309–322. [Google Scholar]
- 39.Tegner MJ, Dayton PK. Ecosystem effects of fishing in kelp forest communities. ICES J Mar Sci. 2000;57:579–589. [Google Scholar]
- 40.Roemer GW, Donlan CJ, Courchamp F. Golden eagles, feral pigs and insular carnivores: How exotic species turn native predators into prey. Proc Natl Acad Sci USA. 2002;99:791–796. doi: 10.1073/pnas.012422499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steneck RM, et al. Kelp forest ecosystems: Biodiversity, stability, resilience, and their future. Environ Conserv. 2002;29:436–459. [Google Scholar]
- 42.Graham MH. Effects of local deforestation on the diversity and structure of Southern California giant kelp forest food webs. Ecosystems (N Y, Print) 2004;7:341–357. [Google Scholar]
- 43.Braje TJ, Erlandson JM, Rick TC, Dayton PK, Hatch MBA. Fishing from past to present: Continuity and resilience of red abalone fisheries on the Channel Islands, California. Ecol Appl. 2009;19:906–919. doi: 10.1890/08-0135.1. [DOI] [PubMed] [Google Scholar]
- 44.Braje TJ, DeLong RL. In: Proceedings of the Seventh California Islands Symposium. Damiani CC, Garcelon DK, editors. Arcata, CA: Institute for Wildlife Studies; 2009. pp. 43–52. [Google Scholar]
- 45.Roemer GW, Coonan TJ, Garcelon DK, Bascompte J, Laughrin L. Feral pigs facilitate hyperpredation by golden eagles and indirectly cause the decline of the island fox. Anim Conserv. 2001;4:307–318. [Google Scholar]
- 46.Coonan TJ, et al. In: Proceedings of the Sixth California Islands Symposium. Garcelon DK, Schwemm CA, editors. Arcata, CA: Institute for Wildlife Studies; 2005. pp. 263–273. [Google Scholar]
- 47.Latta BC, Driscoll DE, Linthicum JL, Jackman RE, Doney G. In: Proceedings of the Sixth California Islands Symposium. Garcelon DK, Schwemm CA, editors. Arcata, CA: Institute for Wildlife Studies; 2005. pp. 341–350. [Google Scholar]
- 48.Collins PW, Latta BC, Roemer GW. Does the order of invasive species removal matter? The case of the eagle and the pig. PLoS One. 2009;4:e7005. doi: 10.1371/journal.pone.0007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coonan TJ. Island fox recovery program San Miguel and Santa Rosa Islands 2007 Annual Report. Ventura, CA: Channel Islands National Park Technical Report 08-01; 2008. [Google Scholar]
- 50.Blasius ME, Goodmanlowe GD. Contaminants still high in top-level carnivores in the Southern California Bight: Levels of DDT and PCBs in resident and transient pinnipeds. Mar Pollut Bull. 2008;56:1973–1982. doi: 10.1016/j.marpolbul.2008.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.