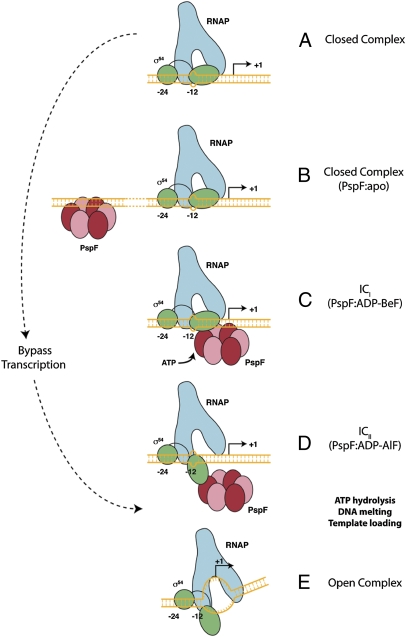
Fig. 4.
Schematic representation of the proposed pathway to open complex formation by Eσ54. (A) The closed complex with inhibitory −12 fork junction structure is potentially engaged by the apo-form of the activator ATPase (here PspF) binding to upstream DNA enhancer sites (B). ICi (C) and ICii (D) represent two different ATP-bound states of the activator ATPase, with ADP-BeF being closer to the ground state than ADP-AlF. Importantly, in (D), the RNAP active site is functional and accessible to single-stranded DNA. We infer that ADP+Pi formation and/or release are required to establish the open complex (E). The properties of the ADP-BeF- (C) and ADP-AlF–dependent (D) complexes are distinct, emphasizing the importance of prehydrolysis states for substrate engagement. These discrete nucleotide-bound states are suggested to reflect different states of the activator ATPase in relation to being poised for hydrolysis—a view supported by recent crystallographic studies of a Rho-RNA complex in which individual catalytic (i.e., ATP hydrolysis) sites were shown to be distinct, some closer to the hydrolysis state than others and each with different modes of RNA binding (5).