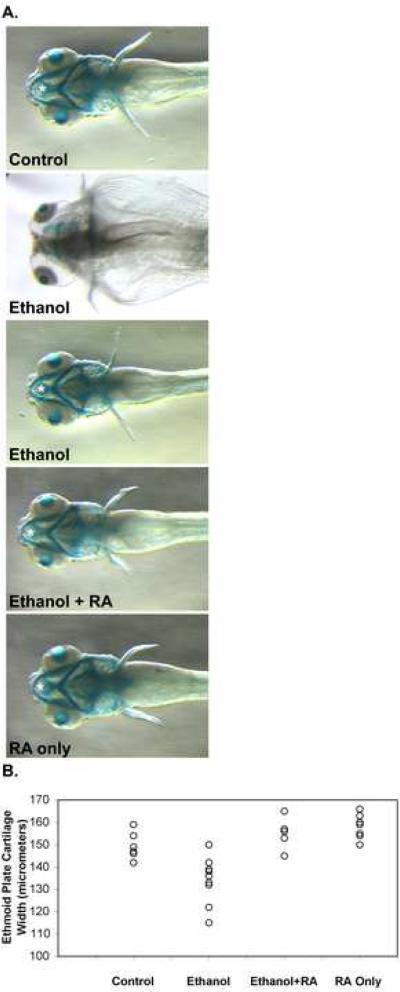
Figure 2.
Ethanol treatment of zebrafish embryos during gastrulation and somitogenesis stages produces craniofacial defects that were improved by including retinoic acid during ethanol treatment. (A.) Dorsal views (anterior is left) of 5 dpf alcian blue stained larvae were imaged using a steromicroscope. Embryos were untreated in control embryos (top panel), and treated with 100 mM ethanol (second and third panels from top), 100 mM ethanol and 10−9 M retinoic acid (third panel from top), and 10−9 M retinoic acid alone (bottom panel) from 3 hpf until 24 hpf (see Materials and Methods). At 24 hpf, ethanol and/or retinoic acid treatments were discontinued, and embryos were incubated with normal embryo medium until 5 dpf. These 5 dpf larvae were processed for alcian blue staining to label craniofacial cartilages. White asterisks mark the ethmoid cartilage measured (in other embryos) for Table I. The ethmoid cartilage cannot be seen in the more severely affected ethanol treated embryo (second panel from top), and consequently, when we could not detect the cartilage, we did not include these embryos in our measurements. Note that the ethmoid cartilage extends beyond the hyoid arch (the most anterior structure in the ventral jaw cartilages). (B.) Scatter plot representation of ethmoid cartilage width variation in embryos treated from 3 hpf until 24 hpf (during gastrulation and somitogenesis) with 100 mM ethanol, 10−9 M retinoic acid, or 100 mM ethanol and 10−9 M retinoic acid together, and compared to untreated control embryos. Ethmoid plate cartilage width was measured in alcian blue stained 4.5 dpf larvae (see Table I for statistical analysis).