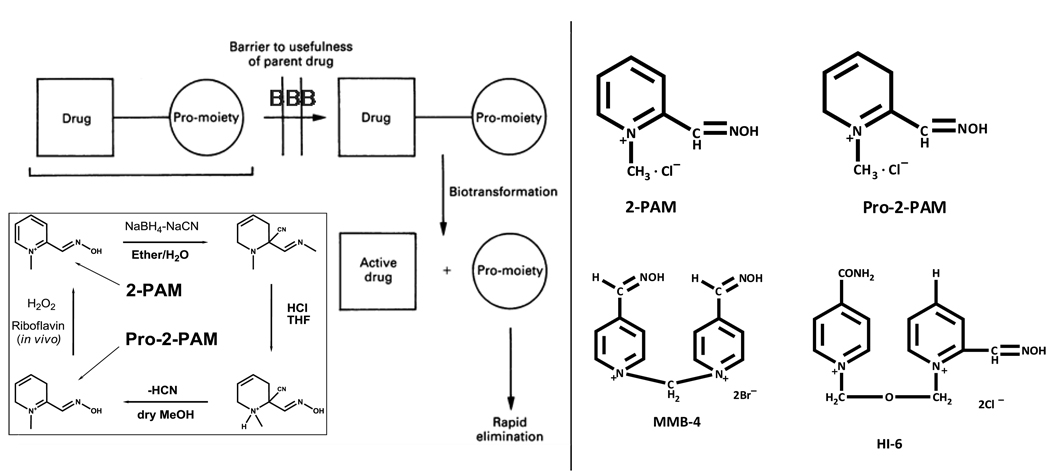
Figure 1.
Pro-2-PAM synthesis and in vivo distribution in comparison to 2-PAM. On the lower left, synthesis of pro-2-PAM from 2-PAM [11]. Pro-2-PAM, a pro-drug form of 2-PAM, exhibits lipid solubility due to its reduced charge, readily penetrates the blood brain barrier to the CNS as well as the PNS. Inside the brain, Pro-2-PAM is rapidly converted to 2-PAM [13] (modified from [12]). 2-PAM, due to its quaternary charged pyridine ring, cannot cross the BBB. Right, structures of 2-PAM, Pro-2-PAM, MMB-4, and HI-6.