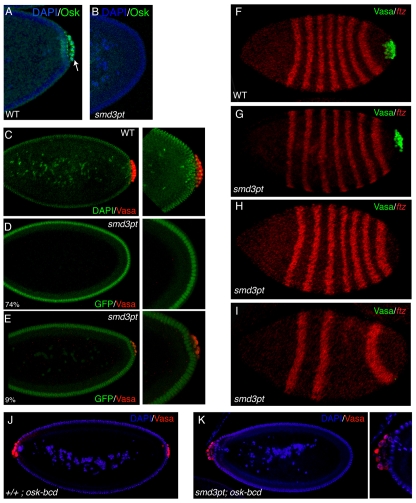
Fig. 7.
SmD3 is required for germ cell specification and embryonic patterning. (A,B) Embryos from wild-type (A) or SmD3pt (B) females were processed for immunofluorescence using an antibody against Oskar (green) and were counterstained with DAPI (blue). In contrast to wild type, most SmD3pt embryos contained neither germ cells nor posterir Oskar. (C-E) Embryos from wild-type (C) or SmD3pt (D-E) females raised at 27°C were processed for immunofluorescence using an antibody against Vasa (red). Wild-type embryos were counterstained with DAPI (green) and the green fluorescence of SmD3pt embryos is indicated. Most SmD3pt embryos (74%) did not contain germ cells (D). A small percentage (9%) of embryos contained many fewer germ cells than the wild type (E). (F-I) Embryos from wild-type (F) or SmD3pt (G-I) females were processed for in situ hybridization using a probe against fushi tarazu (ftz, red) and immunofluorescence using an antibody against Vasa (green). (G) An SmD3pt embryo that contains germ cells and proper patterning. (H) An SmD3pt embryo that lacks germ cells yet retains all posterior segments. (I) An SmD3pt embryo lacking both germ cells and posterior segments. (J,K) Embryos from wild-type (J) or SmD3pt (K) females expressing UAS-osk-bcd driven by nanos-Gal4 were processed for immunofluorescence using an antibody against Vasa (red) and counterstained with DAPI (blue). Although SmD3pt embryos were able to form anterior germ cells (34.3±1.6%), anterior germ cell formation was less efficient than in wild type (52.5±2.5%). These results are significant at P<0.0005.