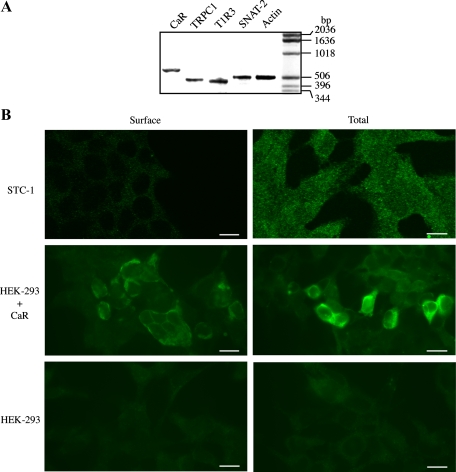
Fig. 3.
A: expression of CaR, transient receptor potential canonical 1 (TRPC1), T1R3 (taste receptor), and Na+-coupled neutral amino acid transporter 2 (SNAT2) in STC-1 cells. RT-PCR was performed using specific primers for each of the mRNAs encoding CaR, TRPC1, T1R3, SNAT2, and actin (see materials and methods) on 5 μg of total RNA isolated from STC-1 cells. PCR products were resolved on 2% agarose, and the gel was then stained with ethidium bromide. Products of the predicted size for the CaR (627 bp), TRPC1 (462 bp), T1R3 (423 bp), SNAT2 (506 bp), and actin (502 bp) were detected only when both antisense (A) and sense (S) primers were included during the second cDNA strand synthesis reactions. The results are representative of two independent experiments. B: intracellular distribution of the CaR in STC-1 cells. Fixed STC-1 cells, either permeabilized (total) or not (surface) using 0.3% Triton X-100, were incubated with an antibody that recognizes the NH2-terminus of the CaR extracellular domain. After extensive washes, the samples were incubated with Alexa Fluor 488-conjugated anti-rabbit and examined with a confocal microscope, as described under materials and methods. As controls, HEK-293 cells transfected (HEK-293 + CaR) or not (HEK-293) with a plasmid encoding the CaR were prepared for immunocytochemistry, as described for STC-1 cells, to detect total and surface CaR expression. The selected cells displayed in the figure were representative of 90% of the population of positive cells. Scale bars, 10 μm.