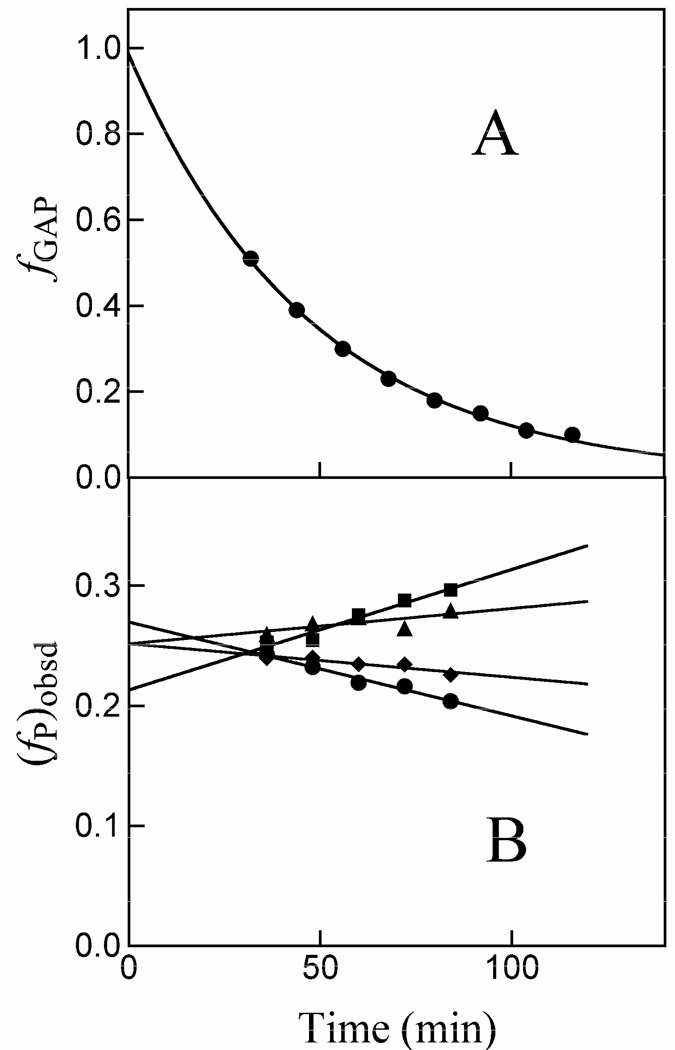
Figure 1.
Data for the reaction of GAP (10 mM) in the presence of 85 µM K12G TIM in D2O at pD 7.9 (10 mM imidazole), 25 °C and I = 0.15 (NaCl) monitored by 1H NMR spectroscopy. A. Timecourse for the first-order disappearance of the substrate GAP. B. Dependence of the observed fractional yields of the products on time. Extrapolation of these data to zero time (solid lines) gave the initial fractional yields of the products of the enzyme-catalyzed and nonenzymatic reactions of GAP, (fP)o or (fMG)tot = (fMG)N + (fMG)E reported in Table 1. (◆), Methylglyoxal; (■), d-DHAP; (▲), DHAP; (●), d-GAP.