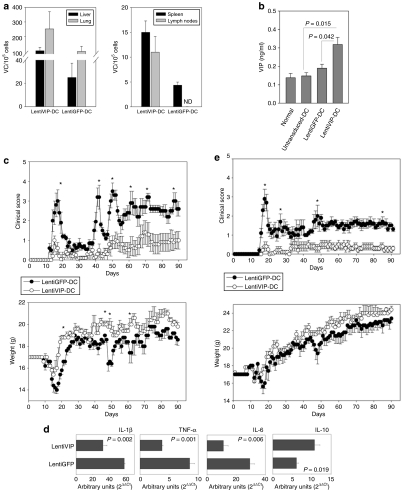
Figure 6.
LentiVIP-DC reduces severity in two experimental autoimmune encephalomyelitis (EAE) models. (a) Liver, lung, spleen, and lymph nodes (axillary and inguinal) were collected 48 hours after intravenous inoculation of transduced cells and genomic DNA was isolated. The presence of LentiVIP-DC and LentiGFP-DC was analyzed by quantitative PCR using primers specific for sequences for each vector. Presented data are after subtraction of background from tissues of control mice. (b) Serum from LentiVIP-DC, LentiGFP-DC, and untransduced-DC SJL-treated mice was obtained 48 hours after the dendritic cell (DC) inoculation and vasoactive intestinal peptide (VIP) levels were measured by enzyme-linked immunosorbent assay and compared with endogenous levels of VIP in serum of healthy mice. (c) SJL/J mice (5 mice/group) immunized with PLP139–151 as described in Methods were inoculated intravenous (tail vein) on day 6 with LentiVIP-DC or LentiGFP-DC (3 × 106 cells) pulsed with 50 µg/ml of PLP (3 × 106). Clinical score and weight was followed daily for >90 days. (d) Total RNA was extracted from spinal cord of LentiVIP-DC and LentiGFP-DC SJL-treated animals at the peak of disease and analyzed for cytokine expression by real-time qPCR. The expression of cytokines was normalized to GAPDH and is relative to healthy controls (no EAE). Degree of significance P < 0.05. (e) C57BL/6 mice (6 mice/group) were immunized with MOG35–55 as described in Materials and Methods. On day 8, the mice were inoculated intravenous with 3 × 106 LentiGFP-DC or LentiVIP-DC pulsed with 50 µg/ml of MOG. Clinical score and weight was followed daily for >90 days. *P < 0.05. IL, interleukin; TNF, tumor necrosis factor.