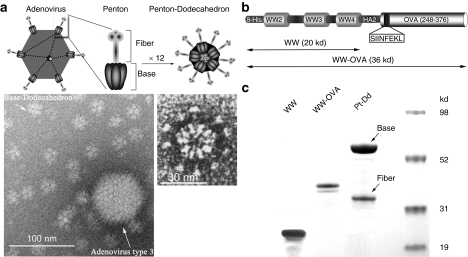
Figure 1.
Description of Pt-Dd VLPs as protein delivery system for vaccine development. (a) Structure of human Ad3 and its Pt-Dd particle. Schematic representation of the Ad morphology, including an icosahedral capsid with Pt structures at the vertices (upper left diagram). Pt (zoomed diagram) comprises a noncovalent complex of trimeric fiber protein attached to a pentameric penton base. Self-association of the Pt results in the formation of the DNA devoid Pt-Dd particle (upper right diagram). Electron micrographs (obtained as described in ref. 18) illustrate the size comparison between Ad3 capsid and Dd particle (left) and the Pt-Dd detailed structure (right). (b) Schematic representation of the recombinant fusion protein WW-OVA, which comprises (i) WW2-3-4 domains from Nedd4; (ii) the NH2-terminal peptide of influenza virus HA2; (iii) a 129 amino acid C-terminal fragment of OVA containing the MHC class I immunodominant peptide SIINFEKL. (c) SDS-PAGE analysis of purified proteins. WW and WW-OVA proteins were expressed in BL21 cells and purified by affinity column. Pt-Dd VLPs were expressed in baculovirus and purified in a sucrose density gradient. Ad3, adenovirus type 3; MHC, major histocompatibility complex; OVA, ovalbumin; Pt-Dd, penton-dodecahedron; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; VLP, virus-like particle.