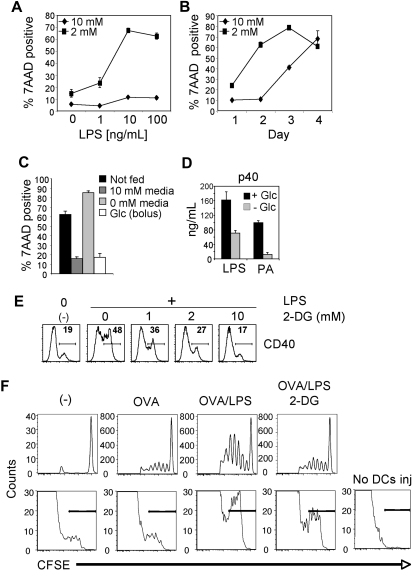
Figure 3.
Glycolysis is essential for cellular activation and survival after exposure to TLR agonists. (A) Survival of DCs 2 days after stimulation with LPS in 10mM or 2mM glucose. (B) Survival of DCs over time after stimulation with LPS in media containing either 10mM or 2mM glucose. Data points represent the percentage of 7-amino-actinomycin D (7AAD)–positive cells as determined by flow cytometry. (C) Survival of DCs after glucose supplementation. DCs were cultured for 2 days in the presence of 10mM glucose. On day 2, the media was either left unchanged (not fed) or supplemented with 10mM glucose (bolus) or replaced with media containing 10 or 0mM glucose. Cell viability was determined 24 hours later by 7AAD uptake. (A-C) Error bars are SDs from triplicate measurements. (D) Cytokine (p40) production by DCs in response to TLR agonists in the presence or absence of glucose. Cytokine production was determined by enzyme-linked immunoabsorbent assay 18 hours after activation. (E) LPS-induced activation of DCs in the presence of the glycolytic inhibitor 2-DG. Flow cytometry was used to measure surface expression of CD40 after LPS stimulation in the presence of the indicated concentrations of 2-DG. Unstimulated DCs cultured in the absence of 2-DG or LPS are shown for comparison (-). Numbers represent the percentage of cells staining positive for CD40. (F top) DO11.10 CD4+ T-cell proliferative responses, measured by CFSE-dilution, in adoptively transferred mice immunized 4 days previously with DCs alone (-), or DCs pulsed with OVA alone, DCs pulsed with OVA plus LPS, or DCs pulsed with OVA plus LPS in the presence of 10mM 2-DG. Plots are gated on KJ126+CD4+ cells. (F bottom) Numbers of CFSE-stained CD11c+ cells in LNs draining sites of injection 2 days previously with CFSE-labeled DCs pulsed with OVA alone, pulsed with OVA plus LPS, or pulsed with OVA plus LPS in the presence of 10mM 2-DG. The panel to the right shows the absence of CFSE signal in control popliteal LNs draining noninjected sites. Bars indicate CFSE-positive cells. All data are representative of 2 to 5 independent experiments.