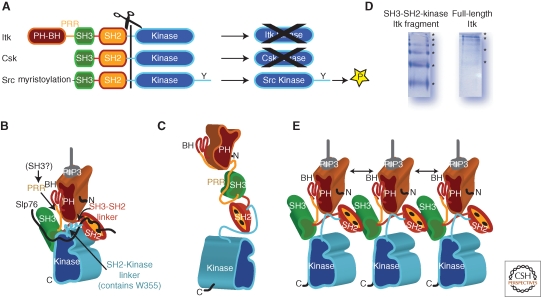
Figure 2.
Activation/inactivation of Itk. (A) Comparison of the domain structures of Itk, Csk and Src kinases. The domains are colored as follows: PH-BH is brown, PRR is yellow, SH3 is green, SH2 is orange, SH2-Kinase linker and Kinase domain are blue. All three kinases share the SH3-SH2-kinase cassette but differ with respect to resulting catalytic activity of the isolated kinase domain. Itk and Csk kinase domains alone show very poor catalytic activity whereas the Src kinase domain readily phosphorylates substrates. (B) Model of active Itk based on biochemical data and the high-resolution structure of Csk (PDB code: 1K9A). The SH2-Kinase linker and SH3-SH2 linker are both arranged along the back of the small kinase lobe. Activating interactions between the kinase domain and the SH2-kinase linker in particular are also supported by a recent structure of Btk (Marcotte et al. 2010). SLP-76 is depicted by the black line and is shown binding to both Itk SH3 and SH2. The PH-BH domain is placed so that its carboxy-terminus is close in space to the amino-terminus of the SH3 domain. The intervening sequence is the PRR, which would be available in this model for binding an SH3 domain from another protein. PH domain is shown bound to its PIP3 ligand. (C) Model of Itk adopting the extended conformation seen for Btk in SAXS studies (Marquez et al. 2003). Of note is the fact that the intramolecular PRR/SH3 interaction is sterically allowed and that the SH2-Kinase linker region is likely in an extended conformation and not the active conformation shown in (B). (D) Native gel electrophoresis for the SH3-SH2-Kinase fragment of Itk and for full length Itk showing ladders indicative of mulitmerization. Sample concentration for both purified proteins is approximately 5 micromolar. (E) Model of Itk multimerization based on the structure of the binary Itk SH3/SH2 complex (PDB code: 2K79). Doubled headed arrows indicate PH domain self-association. Whether Itk multimerization occurs exclusively at the membrane remains to be determined. It is of note that the SH3/SH2 interface defined in PDB 2K79 is accessible in the models shown in both (B) and (C) suggesting that either configuration could multimerize. In (B), (C) and (E) the amino and carboxy-termimi of full length Itk are indicated by N and C, respectively.