Table 2.
Dynamic Kinetic Resolution of 4a
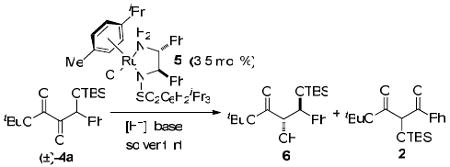 | ||||
|---|---|---|---|---|
| entry | [H−]/base (equiv) | conv (%)a (6 : 2) | erb (anti) | drcanti:syn |
| 1 | HCOOH/NEt3 (3.2/4.4) | 88:12 | 67:33 | 5:1 |
| 2 | HCOOH/NEt3 (12.5/5.0) | 79:21 | 67:33 | 4:1 |
| 3 | HCOOH/NEt3 (3.2/10.0) | 35:65 | 78:22 | 4:1 |
| 4d | HCOONa (5.0) | 97:0 | 67:33 | 3:1 |
| 5e | HCOONa (5.0) | 93:7 | 71:29 | 3:1 |
| 6 | HCOONa (5.0) | 92:8 | 72:28 | 3:1 |
| 7 | HCOOH/DIEA (3.2/10.0) | 67:33 | 75:25 | 2:1 |
| 8 | HCOOH/Cs2CO3 (3.2/10.0) | 91:9 | 70:30 | 3:1 |
| 9 | HCOOH/Li2CO3 (3.2/10.0) | 73:27 | 70:30 | 3:1 |
| 10 | HCOOH/BaCO3 (3.2/10.0) | 46:2 | 70:30 | 3:1 |
| 11 | HCOOH/CdCO3 (3.2/10.0) | 51:5 | 76:24 | 3:1 |
| 12 | HCOOCs (5.0) | 89:6 | 70:30 | 3:1 |
Conversion determined by 1HNMR spectroscopy.
Enantiomeric excess determined by SFC.
Diastereomeric excess determined by 1HNMR spectroscopy.
Reaction run at 0 °C.
Reaction run at 40 °C.
