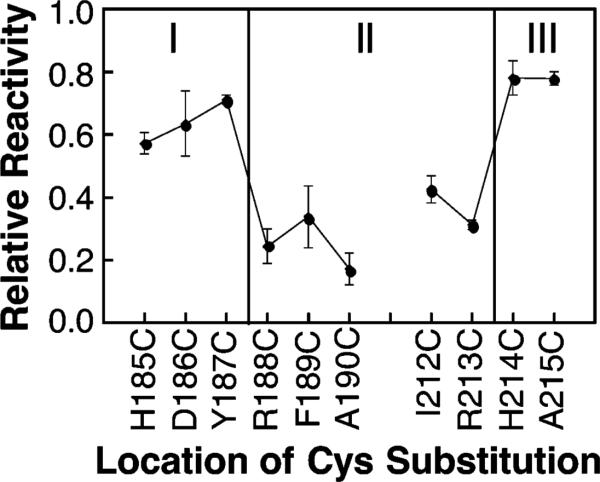
Figure 2.
Relative chemical reactivities of the cysteine substitutions used to map the membrane–water interface onto the surface of the signaling helix. Each indicated mutant receptor possessing a single cysteine per subunit was isolated in native E. coli membranes and reacted with the aqueous alkylating agent 5-iodoacetamidofluorescein (5-IAF) under fixed conditions as described in Materials and Methods. The initial reaction rate was measured in triplicate and normalized to the reaction rate of a fully exposed cysteine residue as previously described for the chemical reactivity scan of the cytoplasmic interface (28). The reactivities of solvent- and headgroup-exposed cysteine residues are significantly higher than those exposed to the bilayer hydrocarbon core (see text).