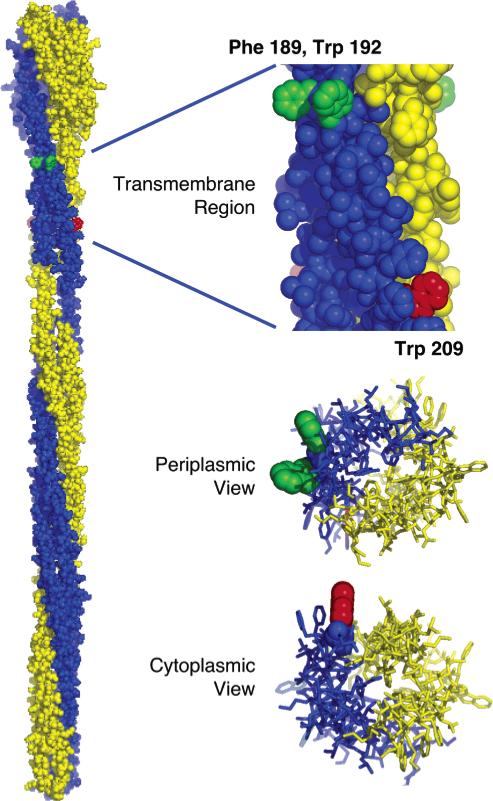
Figure 4.
Model of the full-length receptor structure illustrating the two identical subunits of the homodimer (yellow and blue) (10), built using information provided by crystallographic and chemical studies (4, 7, 25, 32, 33, 38, 39). The expanded views show the transmembrane four-helix bundle as seen from the center of the bilayer (top), the periplasm (middle), and the cytoplasm (bottom). Positions on the signaling helix where side chain substitutions stabilize the receptor on-state (Phe189 and Trp192) or off-state (Trp209) are indicated in green and red, respectively. Notably, the native side chains at these positions project into the surrounding lipids. For simplicity, positions of signal-stabilizing substitutions are highlighted for only one of the two identical subunits. Note that the residue numbers of the model structure (10), which are based on the serine receptor primary sequence, have been replaced with the corresponding residue numbers for the aspartate receptor of the present study. Moreover, the illustrated Phe189 was modeled in place of Gln at the homologous serine receptor position.