Abstract
The etiologic agent of mandibulofacial and maxillofacial abscesses in mice is reportedly coagulase-positive Staphylococcus aureus. Although suggested to be through the oral cavity, the exact route of entry has not been documented. Among the clinical cases of mandibulofacial and maxillofacial abscess we report here, each case that was cultured yielded coagulase-positive S. aureus. Histologically, all of the abscesses examined were directly associated with intralesional hair shafts, both vibrissae and pelage, that were introduced into the submucosa via the maxillary or mandibular molar gingival sulci. Grossly, a variable amount of hair was imbedded in the lingual, buccal, or mesial gingival sulci of the maxillary or mandibular molars or both. Computed tomography revealed that the presence of the hair resulted in inflammation and resorption of alveolar bone. With these findings, we propose that mandibulofacial and maxillofacial abscesses are induced by the mastication and fragmentation of hair ingested during the barbering process. From the resulting foreign body periodontitis, abscess formation originates at the maxillary lingual, buccal, or mesial gingival sulci, resulting in infection of the maxillary molar tooth roots with swelling or rupture through the skin inferior to the eye, or at the mandibular lingual, buccal, and or mesial gingival sulci, resulting in infection of the mandibular molar tooth roots and osteomyelitis with drainage through the skin of the ventral mandible.
Very few cases of mandibulofacial and maxillofacial abscess in mice have been reported in the literature;3,7 these reports discuss the bacteria involved but only speculate on the route of its entry into the oral submucosa. The causative organism in the reported cases3,7 was Staphylococcus aureus,3,7 with coagulase-positive S. aureus specifically mentioned in one case.7 In terms of location, both maxillary and mandibular abscesses were included in one report,3 whereas only mandibular abscesses were reported in the other.7 The current report addresses several cases of mandibulofacial and maxillofacial abscess in mice; in all cases, hair of vibrissae and pelage types were identified within the inflammatory milieu of the gingival sulci of the molars. The etiopathogenesis of mandibulofacial and maxillofacial abscesses is discussed in this retrospective study.
Materials and Methods
All animals examined were housed in a specific pathogen-free, AAALAC-accredited facility in static microisolation caging, with ad libitum nonautoclaved, nonirradiated rodent diet (Harlan Teklad NIH31, Harlan, Indianapolis, IN) and nonacidified, filtered water. The macroenvironmental conditions within all rooms were maintained at 21.1 to 25.6 ºC (70 to 78 °F), 30% to 50% relative humidity, 10 to 15 air changes hourly, and a 12:12-h light:dark cycle. All animal manipulations were performed in a HEPA-filtered cage-changing station (Stay Clean Workbench, Lab Products, Seaford, DE). The mice examined in this study were assigned to existing University of California IACUC-approved animal use protocols of various investigators. Female CD1 mice (age, 3 wk; Charles River Laboratories, Wilmington, MA) were housed at 3 mice per cage and exposed by direct dirty-bedding transfer from no more than 70 cages per sentinel box. Sentinel mice were screened quarterly for mouse hepatitis virus, Sendai virus, pneumonia virus of mice, reovirus 3, mouse encephalomyelitis virus (GDVII serotype), ectromelia virus, Mycoplasma pulmonis, mouse parvovirus, minute virus of mice, rotavirus, and lymphocytic choriomeningitis virus and endo- and ectoparasites.
All of the mice in this study were identified as clinical cases by husbandry technicians and recorded by Animal Health Technicians in REACTor, a Palm Pilot (Palm, Sunnyvale, CA)-based software program written specifically for our institution (Marshall Reed, UCLA). Information collected included facility, room, rack, investigator, protocol number, contact phone extension, laboratory contact person, technician observations, treatments, and veterinarian communications.
Maxillofacial abscesses were identified in various strains of mice with white, agouti, or black coat colors during routine health checks, and the affected mice were euthanized due to their diseased condition. Information on the number of mice per cage and the presence or absence of barbering of cagemates was not recorded. All of the mandibulofacial abscesses occurred in a cluster of 9 129/SvEv and 129/SvEv X C57BL/6 mice from the same investigator, and a complete history was obtained because these cases occurred as a major disease outbreak specifically in this mouse strain. This cluster of mice revealed that animals with mandibulofacial abscesses possessed facial vibrissae whereas cagemates did not; the mice with abscesses thus were diagnosed as the ‘barberers.’
All mice examined in this study were euthanized by carbon dioxide inhalation. After euthanasia, representative abscesses from the maxilla and mandible were cultured for aerobic and anaerobic bacteria (IDEXX, Sacramento, CA). Complete necropsies were performed, and the entire head of each mouse was fixed in 10% neutral buffered formalin for 24 h.
Microcomputed tomography.
After formalin fixation, the head of a mouse was secured in a 50-mL plastic conical centrifuge tube for microcoputed tomograpy (mCT 40, Scanco Medical, Brüttisellen, Switzerland) at 55 kVp, 72 mA, and an integration time of 300 ms with an isotropic voxel size of 30 µm. The digital image was reconstructed by using a Gaussian filter of s = 1 and support = 1 to remove noise. A set threshold of 16.5% of maximal gray scale was used to visualize mineralized tissues. Visualization and reconstruction of the data were performed by using vendor-supplied software (µCT Rat T3.3 and µCT Evaluation Program version 5.0, Scanco Medical). 3D reconstruction based on 30-µm slices precisely reproduced the positions of sutures, notches, and bony tips for accurately defining various anatomic landmarks on target anatomic structures.
Gross examination.
Once the hypothesis was made that all facial abscesses in mice were the result of hair-induced inflammation, the mandibles were separated from the cranium by cutting the mandibular ramus with scissors. The mandibular and maxillary molars then were examined microscopically (DP25 Dissection Microscope, Olympus, Center Valley, PA). Images were collected by using a digital camera (ELS Digital Rebel, Cannon, Lake Success, NY).
Radiography.
The mandibles from 2 of the mice with mandibular abscesses were dissected from the skull and radiographed (70 kVp, 7 mA; Oralix 70 Densomat Dental Radiograph Machine, Philips, New York, NY). The radiographs were developed automatically (AT 2000 Plus, Air Techniques, Corona, CA).
Histologic examination.
After formalin fixation, all skulls and mandibles were decalcified for 24 h in EDTA and hydrochloric acid (Protocol Decalcifier B, Fisher Scientific, Pittsburgh, PA). The heads then were sliced transversely to form multiple thin sections that progressed from the planum nasal to the brain stem. All sections were embedded in paraffin, and 5-µm sections were stained with hematoxylin and eosin (Pathology, Torrance, CA).
Results
This study encompasses clinical cases of rodents that were reported in REACTor from 21 through 29 December 2010. Of the 47,814 health cases reported during that period, 139 (0.3%) were facial abscesses. Although precise numbers based on location are not available, the maxillofacial presentation was the more common among the abscesses examined at necropsy. In the subset of cases examined histologically for this study, 30 abscesses were maxillofacial and 9 were mandibulofacial.
To define the etiopathogenesis of facial abscesses in mice, clinical cases of suspected facial abscess were identified during clinical rounds of the animal facilities. Once the clinical veterinarian evaluated and confirmed the diagnosis of facial abscess by anesthetizing the mouse with isoflurane and lancing the swelling, cases were classified as maxillofacial if the swelling was infraorbital (Figure 1 A) and as mandibulofacial if swelling with or without drainage was present in the area of the ventral ramus of the mandible (Figure 1 B). By both gross and histologic examination, all mandibulofacial and maxillofacial abscesses were associated with hair. Two types of hairs were identified histologically: hairs with thick, pale green, light refractive walls and hollow cores (representative of vibrissae) and solid hairs often with pigmented central cores (representative of pelage hair). Compared with mice with no periodontal disease (Figure 2 A), gross examination demonstrated that vibrissae and pelage hairs penetrated and expanded the gingival sulci of the mandibular and maxillary molars of abscessed mice (Figure 2 B).
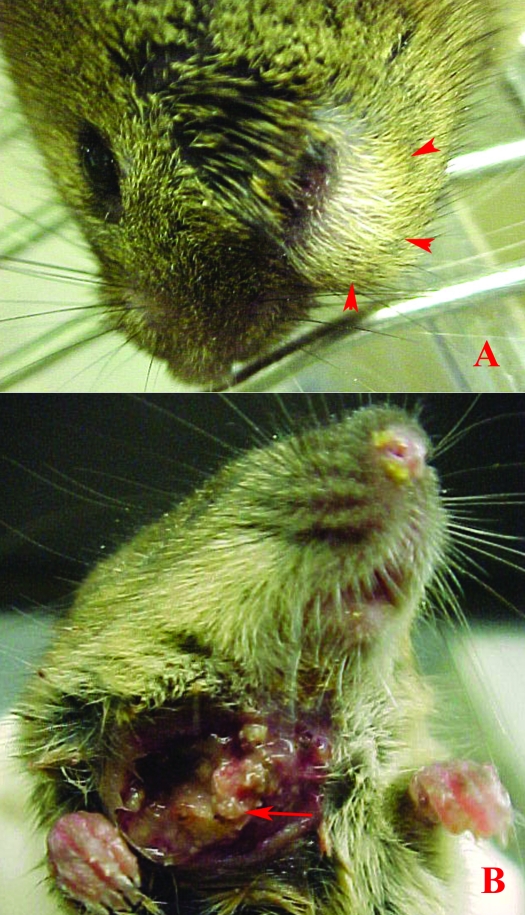
Figure 1.
(A) Clinical presentation of a typical maxillofacial abscess in a mouse, with swelling inferior to the eye (arrowheads). (B) Clinical presentation of a mandibulofacial abscess in a mouse, with drainage ventral to the mandibular ramus. Note the multifocal, well-defined necrotic nodules of the botryoid abscess (arrow).
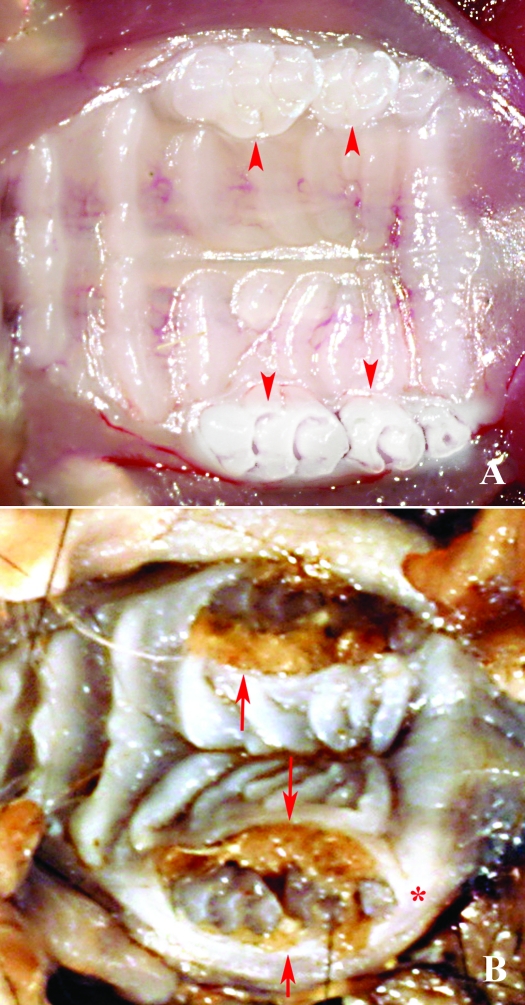
Figure 2.
(A) Maxillary molar arcade of a mouse with normal dentition and tight gingival sulci (arrowhead). (B) Maxillary molar arcade demonstrating extreme expansion of the lingual and buccal gingival sulci with hair. Note the separation and rotation of M1 and M2 and the distance between M2 and M3 in the arcade (*).
The maxillofacial and mandibulofacial abscesses were similar histologically, except that mandibulofacial abscesses often resulted in severe osteomyelitis. Regardless of abscess location, hair shafts penetrated the oral mucosa through the buccal, lingual, or mesial gingival sulci of one or multiple mandibular or maxillary molars. Both grossly and histologically abscesses were composed of well-defined, multilobulated, botryoid lobules separated and supported by thick bands of dense fibrous connective tissue (Figure 3 A, B). Histologically, each individual lobule contained large numbers of neutrophils, clusters of coccoid bacteria, and central Splendore–Hoeppli material (Figure 3 B). Within the center of many of the abscess lobules were single to multiple, linear, homogeneous, pale amphophylic crystalline materials similar to but with greater thickness than those noted in cases of eosinophilic crystalline pneumonia (not shown). Osteolysis was a common finding in all cases of abscess regardless of location and was identified as scalloping of the bone periphery to extensive gaps or discontinuations of bone. In several cases, inflammation, hair, and bacteria traversed through the orbital portion of the frontal bone through these gaps, and filled the retroorbital space and (or) directly affected the Hardarian gland, resulting in Hardarian adenitis and necrosis with or without ocular proptosis.
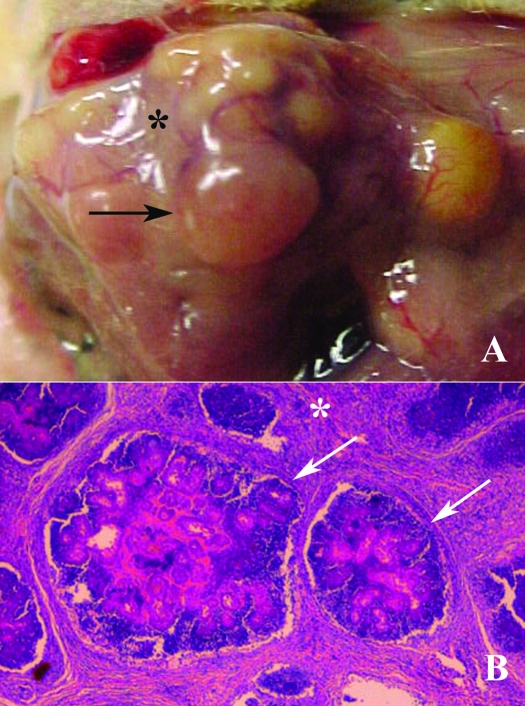
Figure 3.
A: Gross examination: Well-defined botryoid abscesses (arrows) separated by various amounts of connective tissue (*), typical of Staphylococcus aureus infections. (B) Histologically, the abscess is multilobulated (arrow) and separated by bands of dense fibrous connective tissue (*). The central areas contain neutrophils, bacterial colonies, and Splendore–Hoeppli material.
Compared with mice with no oral or periodontal lesions, radiographs of the mice with mandibulofacial abscesses demonstrated severe multifocal osteolysis and severe exostosis of the body of the mandible (Figure 4 A, B) resembling ‘lumpy jaw’ in ruminants. Histologically, these lytic areas were filled with neutrophils, bacterial colonies, central Splendore–Hoeppli material and crystals similar to those previously described. In some cases, the tongue adjacent to the mandibular abscess was ulcerated and infected secondary to penetrating hair shafts.
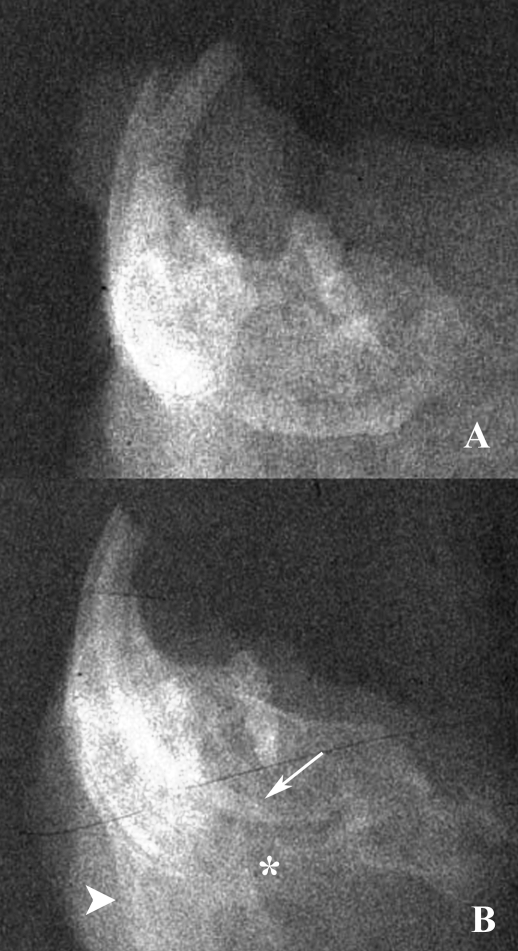
Figure 4.
A. Radiograph of a mandible from a mouse with no periodontal disease. (B) Radiograph demonstrating irregular periosteal proliferation (large arrow head) and severe osteolysis (*). Note the length of the incisive root (small arrowhead).
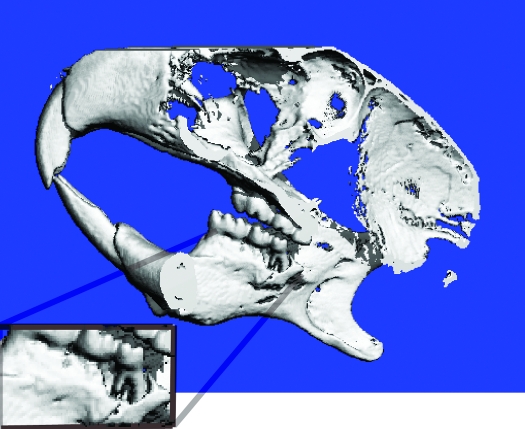
Microcomputed tomography of a mouse with a moderately severe mandibulofacial abscess demonstrated severe bilateral bone lysis extending around the buccal and lingual surfaces of the molars. In this case, bone resorption was most severe on the buccal surface and resulted in extreme periodontal alveolar bone loss of M3 (Figure 5 A).
Figure 5.
CT scan of the lateral skull of a mouse with a mandibulofacial abscess that did not result in osteomyelitis. Note the extreme periapical alveolar bone loss around the mandibular incisor tooth root (inset).
Discussion
The purpose of this survey was to define the etiopathogenesis of mandibulofacial and maxillofacial abscesses in mice. Mandibulofacial and maxillofacial abscesses at the Division of Laboratory Animal Medicine at the University of California at Los Angeles have presented with abscess formation with intralesional hair originating at either the (1) lingual, buccal, or mesial maxillary gingival sulci, resulting in infection of the maxillary molar tooth roots with swelling or rupture through the skin inferior to the eye or (2) at the lingual, buccal, or mesial mandibular gingival sulci, resulting in infection of the mandibular molar tooth roots with or without osteomyelitis leading to drainage through the skin of the ventral mandible. Of the clinical mandibulofacial and maxillofacial abscesses submitted for bacterial analysis, all were culture-positive for coagulase-positive S. aureus.
According to earlier reports, the entry of bacterial organisms and formation of mandibulofacial and maxillofacial abscesses in barrier-reared mice were thought to be associated with transmission by technicians and other humans, who brought the organisms into the barrier in their oral or nasal secretions.2 However, culturing of the nasal cavities and throats of staff working within an SPF facility where mice with maxillofacial and mandibulofacial abscesses were identified revealed that the S. aureus organisms cultured from the workers were of a different phage type than those that had caused the infections in their mice.3 In addition, oral administration of S. aureus bacteria collected from the humans failed to establish infection in 6-wk-old mice.3 However, the authors of both reports3,7 agreed that the S. aureus organisms cultured from the mandibulofacial and maxillofacial abscesses in their facilities likely entered through the oral cavities of the mice.
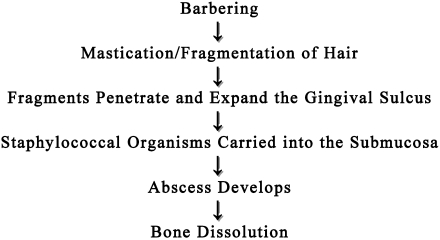
The current report provides evidence that mandibulofacial and maxillofacial abscesses in mice occur secondary to barbering, mastication, and fragmentation of hair that then penetrate into the oral submucosa through the gingival sulcus, resulting in bacterial colonization by S. aureus (Figure 6). Multilobular or botryoid abscesses with Splendore–Hoeppli material and thick connective-tissue capsules are characteristic of tissue reactions induced by coagulase-positive S. aureus.11 Staphylococcus organisms are considered opportunistic and are common surface bacteria that have been recovered from the skin, nasopharynx, and gastrointestinal tract of healthy animals.1
Figure 6.
Proposed etiopathogenesis of mandibulofacial and maxillofacial abscesses in mice.
Although mice with white, agouti, and black coat colors were identified with facial abscesses in the current report, C57BL/6 mice and mice with C57BL/6 background tend to comprise the majority of mice in our facility with periodontitis secondary to hair impaction.5 C57BL/6 mice have exhibited social-dominance barbering, sexual overgrooming, and maternal barberin8,14 as well as anxiety-based barbering associated with overcrowding.10
In all cases (clinical and subclinical) of mandibulofacial and maxillofacial abscess reported here, osteolysis was a prominent histologic feature. S. aureus infections are associated with rapid bone resorption linked to S. aureus surface-associated proteins. Using a murine calvarial bone resporption assay, several investigators demonstrated that these bacterial proteins stimulate fibroblasts or monocytes to release osteolytic cytokines (IL1, TNF).9 In addition, S. aureus binds to bone by means of bone sialoproteins.13 Because all types of abscess examined in the current study had hair-induced inflammation and fibrosis, all components necessary for inducing bone lysis were present, namely inflammatory cells, fibroblasts, and S. aureus organisms. In addition, inflammation is known to activate nuclear IKβ kinase, which is required for osteoclast formation as well as the protection of osteoclasts and their progenitors from TNFα-induced apoptosis.12 Therefore, the imbalance between osteogenesis and osteoclasis results in an overall loss of bone.
The clinical presentation for each of the 2 types of abscess discussed is dependent on the site of tissue penetration by the hair. Abscesses drain toward the area of least resistance. When hair penetrates the gingival sulci of the maxillary molars, the developing abscess results in swelling inferior to the eye and eventually ruptures through the overlying skin. A similar abscess manifestation is noted in dogs with slab fractures of the fourth premolar (or carnassial tooth).4 When hair penetrates the gingival sulci between mandibular molars, staphylococcal bacteria can be introduced deep into the dental alveolus and become embedded within the mandible or exit lateral to the ramus. Proliferation of bacteria can result in regional osteolysis of the lateral mandible or severe medullary osteolysis and periosteal exostosis, which are characteristic of the condition known as lumpy jaw, which occurs in many types of hoof stock.6,15 Lumpy jaw, caused by Actinomyces bovis,15 occurs in a very similar way to the condition in mice, except that the bacteria are introduced by stiff feed stuffs (rather than hair) that penetrate the mucosa by means of the gingival sulcus. Eventually, the abscess penetrates the mandibular cortical bone and drains to the area of least resistance, which is typically through the ventral mandibular skin.
In conclusion, the proposed etiopathogenesis of mandibulofacial and maxillofacial abscesses in mice is as follows: excessive barbering or grooming activities leads to the mastication and fragmentation of hair, which then becomes entrapped and impacted in the gingival sulcus of the molar teeth. The hair acts as a foreign body, resulting in ulceration and inflammation of the periodontium and carries commensal coagulase-positive S. aureus bacteria deep into the submucosa. The current findings suggest the following 2 outcomes depending on the location of hair penetration and bacterial invasion: abscess formation originating at either the (1) maxillary lingual, buccal, or mesial gingival sulci resulting in infection of the maxillary molar tooth roots, with swelling or rupture through the skin inferior to the eye, or (2) mandibular lingual, buccal, or mesial gingival sulci, resulting in infection of the mandibular molar tooth roots with or without osteomyelitis, leading to drainage through the skin of the ventral mandible.
Acknowledgments
I thank Dr Ichiro Nishimura (School of Dentistry, UCLA) for his technical assistance with the CT scan and images, Luis Papa (UCLA DLAM Necropsy Technician), all of the animal care technicians for identifying the health cases used in this study, and the residents and colleagues that reviewed the manuscript prior to its submission.
References
- 1.Besch-Williford CL, Wagner JE, Lindsey JR, Shimizu A. 2002. Staphylococcosis, p 93–94 : Fox JG, Anderson LC, Lowe FM, Quimby FW. Laboratory animal medicine. San Diego (CA): Academic Press [Google Scholar]
- 2.Blackmore DK, Francis RA. 1970. The apparent transmission of staphylococci of human origin to laboratory animals. J Comp Pathol 80:645–651 [DOI] [PubMed] [Google Scholar]
- 3.Clarke MC, Taylor RJ, Hall GA, Jones PW. 1978. The occurrence in mice of facial and mandibular abscesses associated with Staphylococcus aureus. Lab Anim 12:121–123 [DOI] [PubMed] [Google Scholar]
- 4.DeBowes LJ. 2003. Small animal dental diseases, p 310–317 In: Morgan RV, Bright RM, Swartout MS. Handbook of small animal practice. Philadelphia (PA): Saunders [Google Scholar]
- 5.Duarte-Vogel S, Lawson G. 2010 Unpublished data. [Google Scholar]
- 6.Fagan DA, Oosterhuis JE, Benirschke K. 2005. Lumpy jaw in exotic hoof stock: a histopathologic interpretation with a treatment proposal. J Zoo Wildl Med 36:36–43 [DOI] [PubMed] [Google Scholar]
- 7.Grant N, Jackson K, Lee R, Scharf B. 2002. Mandibular mass in a young Swiss-Webster mouse. Lab Anim (NY) 31:25–26 [DOI] [PubMed] [Google Scholar]
- 8.Kalueff AV, Minasyan A, Keisala T, Shah ZH, Tuohimaa P. 2006. Hair barbering in mice: implications for neurobehavioural research. Behav Processes 71:8–15 [DOI] [PubMed] [Google Scholar]
- 9.Nair S, Song Y, Meghji S, Reddi K, Harris M, Ross A, Poole S, Wilson M, Henderson B. 1995. Surface-associated proteins from Staphylococcus aureus demonstrate potent bone-resorbing activity. J Bone Miner Res 10:726–734 [DOI] [PubMed] [Google Scholar]
- 10.Nicholson A, Malcolm RD, Russ PL, Cough K, Touma C, Palme R, Wiles MV. 2009. The response of C57BL/6J and BALB/cJ mice to increased housing density. J Am Assoc Lab Anim Sci 48:740–753 [PMC free article] [PubMed] [Google Scholar]
- 11.Percy DH, Barthold SW. 2001. Staphylococcal infections, p 46–71 : Press IS. Pathology of laboratory rodents and rabbits. Ames (IA): Blackwell Publishing Company [Google Scholar]
- 12.Ruocco MG, Maeda S, Park JM, Lawrence T, Hsu LC, Cao Y, Schett G, Wagner EF, Karin M. 2005. IKβ kinase (IKK)β, but not IKKα, is a critical mediator of osteoclast survival and is required for inflammation-induced bone loss. J Exp Med 201:1677–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryden C, Yacoub A, Maxe I, Heinegard D, Oldberg A. 1989. Specific binding of bone sialoprotein to Staphylococcus aureus isolated from patients with osteomyelitis. Eur J Biochem 184:331–336 [DOI] [PubMed] [Google Scholar]
- 14.Sarna JR, Dyck RH, Whishaw IQ. 2000. The Dalila effect: C57BL6 mice barber whiskers by plucking. Behav Brain Res 108:39–45 [DOI] [PubMed] [Google Scholar]
- 15.Smith BP. 2002. Actinomycosis (lumpy jaw), p 699–700 : Smith BP. Large animal internal medicine. St Louis (MO): Mosby [Google Scholar]