Fig. 6.
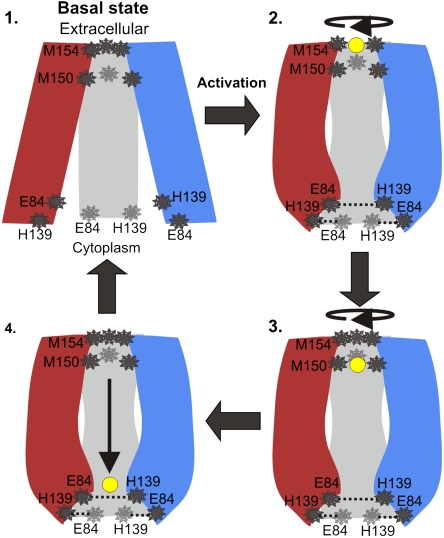
Transport mechanism. In the proposed mechanism the copper ions are transported one at a time, and the TM region of hCTR alternates between four conformations. The three subunits are shown in red, blue, and gray, with residues of interest depicted in dark gray and the copper ion in yellow. Step 1: In the basal state, ions do not bind to the TM domain, the Met154 triad serves as an external gate and (unprotonated) His139 does not interact with Glu84. Our model structure is suggested to depict the basal conformation, because it was computed from a cryoEM map determined at pH 7.4 (9), a condition in which hCTR1 exhibits low activity according to Lee et al. (8). Step 2: Activation resulting, for example, from pH shift or ion binding, involves conformation change at the cytoplasmic end, along with a rotational movement at the extracellular end. A copper ion binds to the Met154 triad, and (protonated) His139 interacts with Glu84, stabilizing the conformation. Step 3: Following another conformational change at the extracellular end, the copper ion is passed on to the Met150 triad and the Met154 closes the pore entrance, thereby preventing the entry of a second ion. Step 4: The copper ion passes freely through the polar pore. It is yet to be revealed how copper ions are passed through to the C-terminus and from there to the copper chaperones.