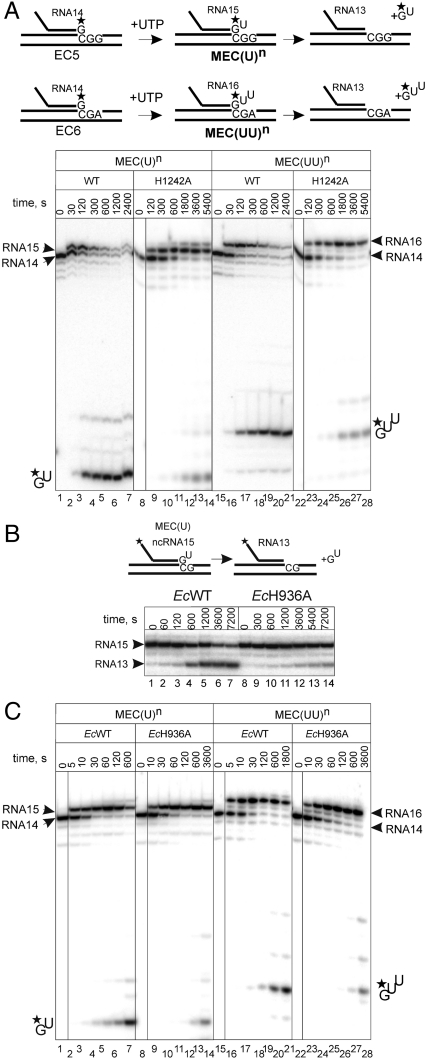
Fig. 4.
Histidine of the TL is required for RNA hydrolysis irrespective of the extent of elongation complex backtracking, and this function of the TL is conserved among bacteria. (A) Schematics at the top of the panel shows formation of elongation complexes MEC(U)n backtracked by 1 bp [formed from elongation complex EC6 (Fig. S1)] and MEC(UU)n backtracked by 2 bp [formed from elongation complex EC5 (Fig. S1)]. Below: Kinetics of hydrolysis of the second and the third phosphodiester bonds in MEC(U)n and MEC(UU)n, respectively, formed via 1 mM UTP misincorporation by WT and H1242A RNAPs. A black vertical line separates lanes originating from the same gel that were brought together. (B) Hydrolysis of the second phosphodiester bond in assembled MEC(U) by E. coli EcWT and EcH936A RNAPs. (C) Kinetics of hydrolysis of the second and the third phosphodiester bonds in MEC(U)n and MEC(UU)n, respectively, formed via 1 mM UTP misincorporation by E. coli EcWT and EcH936A RNAPs (see scheme of the reactions in A). A black vertical line separates lanes originating from the same gel that were brought together.