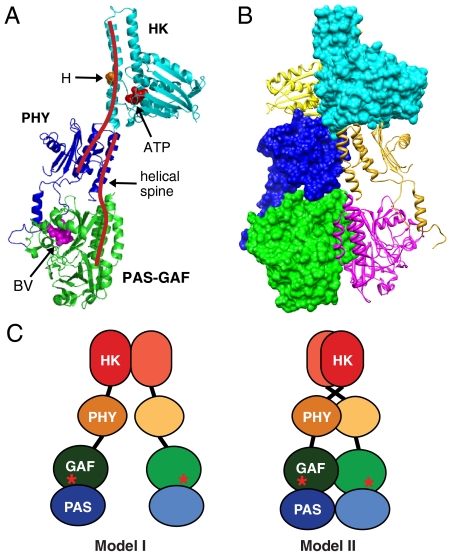
Fig. 4.
Proposed model of the DrBphP dimer derived from both cryoEM and crystal structures. (A) Ribbon diagram of the DrBphP monomer assembled based on cryoEM from the crystal structures of the PAS-GAF domains (green) from DrBphP (13), the PHY domain (purple) from PaBphP (15), and the HK domain (cyan) from T. maritima HK853 (10). The positions of BV, the histidine (H) and ATP-binding site involved in autophosphorylation, and the helical spine are indicated. (B) Composite ribbon and space filling views of the DrBphP dimer showing the right-hand twisted association of the monomers. (C) The previous model (I) and our updated model (II) of Phy dimerization. Astericks indicate BV.