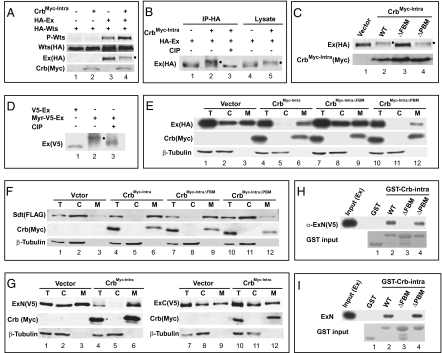
Fig. 5.
Crb binds to Ex through its FBM. (A–D) Hyperphosphorylated Ex is indicated by a filled circle next to the protein band. (A) CrbMyc-Intra promotes Wts phosphorylation in conjunction with Ex. S2R+ cells expressing the indicated constructs were probed with the indicated antibodies. Note the increased P-Wts signal in lane 4 compared with lanes 2 and 3. (B) HA-Ex immunoprecipitates were treated with or without Alkaline Phosphatase (CIP) (lanes 1–3). Note the reversal of CrbMyc-Intra-induced Ex mobility shift by CIP treatment. Ex in S2R+ cell lysates also showed Crb-induced mobility shift (lanes 4–5). (C) The FBM, but not the PBM, is required for CrbMyc-Intra to promote Ex phosphorylation. (D) Membrane targeting of Ex via fusion with a myristylation signal induced Ex phosphorylation (lanes 1–2), which was reversed by CIP treatment (lane 3). (E) The FBM is required for CrbMyc-Intra to recruit Ex to plasma membrane. S2R+ cells expressing HA-Ex with the indicated constructs were subjected to cell fractionation. Cytosol (C), membrane (M), and a portion of the total lysate (T) were probed with indicated antibodies. Note that CrbMyc-Intra (lanes 4–6) or CrbMyc-IntraΔPBM (lanes 10–12), but not CrbMyc-IntraΔFBM (lanes 7–9), relocated Ex from cytosol to membrane (compare with lanes 1–3). (F) Similar to (E) except that Sdt was analyzed. Note that CrbMyc-Intra (lanes 4–6) or CrbMyc-IntraΔFBM (lanes 7–9), but not CrbMyc-IntraΔPBM (lanes 10–12), relocated Sdt from cytosol to membrane (compare with lanes 1–3). (G) CrbMyc-Intra interacts with Ex's N-terminal half (ExN: aa1-709; lanes 1–6) but not with Ex's C-terminal half (ExC: aa710-1427; lanes 7–12) in recruitment assay. (H) Physical association between Crb and Ex in vitro. Cell lysates containing V5-ExN were incubated with glutathione beads containing purified GST (as a control), GST-Crbintra, GST-CrbintraΔFBM or GST-CrbintraΔPBM. V5-ExN associated with the beads was probed with α-V5 antibody. Note that GST-Crbintra and GST-CrbintraΔPBM bound to Ex, but GST and GST-CrbintraΔFBM did not. (I) Purified Crb and Ex bind to each other in vitro. Similar to H except that bacterially purified Myc-ExN was incubated with glutathione beads containing the respective GST fusion proteins, and Ex associated with the beads (pull-down) was probed with α-Myc antibody. Note that GST-Crbintra and GST-CrbintraΔPBM bound to Ex, but GST and GST-CrbintraΔFBM did not.