Fig. 3.
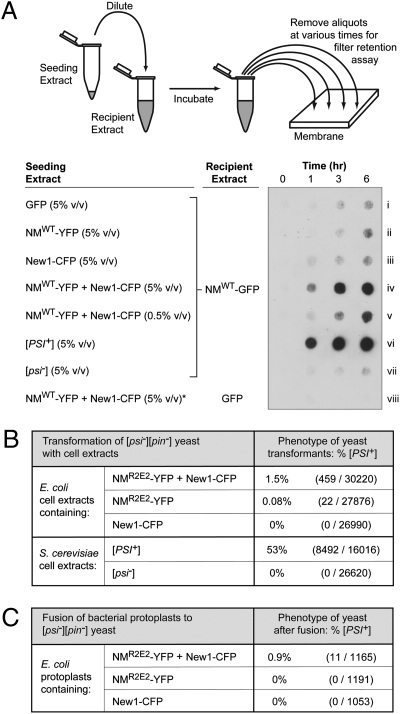
Cells with both NM-YFP and New1-CFP aggregates contain seeding-competent, infectious material. (A) E. coli cell extracts containing SDS-soluble NM-GFP were seeded with E. coli cell extract containing overproduced GFP, overproduced NM-YFP, overproduced New1-CFP, or overproduced NM-YFP and New1-CFP; yeast cell extract prepared from either a [PSI+] or a [psi–] strain was also used as seed (strains YJW96 and SG775, respectively). A seed-only control sample (*) consisted of E. coli cell extract containing overproduced NM-YFP and New1-CFP diluted into E. coli extract containing overproduced GFP only. Cartoon depicts experimental protocol. Samples from seeded reactions were removed at various time points, treated with 2% SDS, and filtered through a cellulose acetate (low-binding) membrane. SDS-stable aggregates that were retained were probed with anti-NM antibody. Extracts were examined for seeding activity in three experiments, with similar results. (B) Infection of [pin–][psi–] yeast spheroplasts with extract prepared from E. coli cells containing the indicated fusion proteins, or with extract prepared from either [PSI+] or [psi–] yeast cells (strains YJW96 and SG775, respectively). The New1-CFP fusion protein was encoded on the chromosome under the control of an IPTG-inducible promoter, and NMR2E2-YFP fusion protein was encoded on a plasmid under the control of an arabinose-inducible promoter. Extracts were prepared from cells 6.5 h after induction of fusion protein synthesis with IPTG and arabinose. Yeast spheroplasts were cotransformed with a yeast shuttle vector containing a URA+ selectable marker. [PSI+] transformants exhibited primarily a “strong” phenotype (3). (C) Fusion of [pin–][psi–] yeast spheroplasts with protoplasts prepared from E. coli cells containing the indicated fusion proteins. The New1-CFP fusion protein was encoded on the chromosome under the control of an IPTG-inducible promoter; the NMR2E2-YFP fusion protein was encoded on a plasmid under the control of an arabinose-inducible promoter. Protoplasts were prepared 6.5 h after induction of fusion protein synthesis with IPTG and arabinose. E. coli cells also contained a yeast shuttle vector with a URA+ selectable marker. Analysis of these data by Fisher's exact test suggests that the observed difference in the frequencies of [PSI+] transformants is statistically significant (P = 10−5). All [PSI+] transformants exhibited a “strong” phenotype (3) (Fig. S4).