Abstract
Hypoxic injury to cardiomyocytes is a stress that causes cardiac pathology through cardiac-restricted gene expression. SRF (serum-response factor) and myocardin are important for cardiomyocyte growth and differentiation in response to myocardial injuries. Previous studies have indicated that AngII (angiotensin II) stimulates both myocardin expression and cardiomyocyte hypertrophy. In the present study, we evaluated the expression of myocardin and AngII after hypoxia in regulating gene transcription in neonatal cardiomyocytes. Cultured rat neonatal cardiomyocytes were subjected to hypoxia, and the expression of myocardin and AngII were evaluated. Different signal transduction pathway inhibitors were used to identify the pathway(s) responsible for myocardin expression. An EMSA (electrophoretic mobility-shift assay) was used to identify myocardin/SRF binding, and a luciferase assay was used to identify transcriptional activity of myocardin/SRF in neonatal cardiomyocytes. Both myocardin and AngII expression increased after hypoxia, with AngII appearing at an earlier time point than myocardin. Myocardin expression was stimulated by AngII and ERK (extracellular-signal-regulated kinase) phosphorylation, but was suppressed by an ARB (AngII type 1 receptor blocker), an ERK pathway inhibitor and myocardin siRNA (small interfering RNA). AngII increased both myocardin expression and transcription in neonatal cardiomyocytes. Binding of myocardin/SRF was identified using an EMSA, and a luciferase assay indicated the transcription of myocardin/SRF in neonatal cardiomyocytes. Increased BNP (B-type natriuretic peptide), MHC (myosin heavy chain) and [3H]proline incorporation into cardiomyocytes was identified after hypoxia with the presence of myocardin in hypertrophic cardiomyocytes. In conclusion, hypoxia in cardiomyocytes increased myocardin expression, which is mediated by the induction of AngII and the ERK pathway, to cause cardiomyocyte hypertrophy. Myocardial hypertrophy was identified as an increase in transcriptional activities, elevated hypertrophic and cardiomyocyte phenotype markers, and morphological hypertrophic changes in cardiomyocytes.
Keywords: angiotensin II, cardiomyocyte hypertrophy, extracellular-signal-regulated kinase (ERK), myocardin, serum-response factor, transcriptional activity
Abbreviations: ANF, atrial natriuretic factor; AngII, angiotensin II; AT1R, AngII type 1 receptor; ARB, AT1R blocker; BNP, B-type natriuretic peptide; DMEM/F12, DMEM (Dulbecco's modified Eagle's medium)/Ham's F12; EMSA, electrophoretic mobility-shift assay; ERK, extracellular-signal-regulated kinase; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GSK, glycogen synthase kinase; JNK, c-Jun N-terminal kinase; mAb, monoclonal antibody; MAPK, mitogen-activated protein kinase; MHC, myosin heavy chain; PI3K, phosphoinositide 3-kinase; siRNA, small interfering RNA; SRF, serum-response factor; VSMC, vascular smooth muscle cell
INTRODUCTION
The heart responds to different stress signals, including biomechanical stress, tissue injury, oxidative stress or neurohumoral activation, and this response may result in hypertrophic growth as indicated by an increase in cardiomyocyte size and protein content, cytoskeleton re-organization and expression of fetal cardiac genes [1–3]. Sustained cardiac hypertrophy may finally lead to cardiomyopathy, heart failure, arrhythmia and even sudden cardiac death, which contribute to a major cause of morbidity and mortality [4,5]. Hypoxia elicits a variety of functional responses in cardiomyocytes, including cell proliferation, cell hypertrophy and cell death. Cardiomyocytes respond to hypoxia to maintain homoeostasis by expressing a number of genes, which are induced by a variety of signalling cascades [1–4]. However, the detailed signalling mechanisms activated in cardiomyocytes in response to hypoxia remain unclear. Understanding the oxygen-sensitive pathways in cardiomyocytes might help in the development of therapies for hypoxia-induced cardiovascular diseases.
Myocardin, a recently discovered novel and potent transcriptional cofactor, co-operates with SRF (serum-response factor) and plays an important role in the gene regulation of cardiac and smooth muscle cell growth and differentiation [6–13]. Previous studies have indicated that forced expression of myocardin induces cardiomyocyte hypertrophy [14–17]. Other studies have also indicated that pressure overload or biomechanical stress may stimulate hypertrophic signals [i.e. ANF (atrial natriuretic factor) and BNP (B-type natriuretic peptide)] and result in cardiomyocyte hypertrophy through increased transcriptional activities of myocardin/SRF in cardiomyocytes [15]. Increased myocardin expression in failing aging human and porcine myocardium compared with healthy individuals has been identified [18]. Myocardin is also up-regulated in aging and end-stage failing human hearts. Fibroblasts from post-myocardial infarction scars acquiring properties of cardiomyocytes after transduction with the recombinant myocardin gene has been another promising finding [16]. According to previous studies [19,20], myocardin transcription and protein levels are increased by hypertrophic stimuli, which may account for the increase in myocardin-dependent gene expression, and myocardin activity is stimulated by hypertrophic signalling, most probably through post-translational modification.
Hypoxic/ischaemic injury is another major stress to the heart, which may lead to cardiomyocyte pathology through fetal cardiac gene expression and specific signal transduction pathways. Previous studies have indicated that hypoxia in cardiomyocytes results in cardiomyocyte hypertrophy and remodelling, which was blocked by ARBs {AT1R [AngII (angiotensin II) type 1 receptor] blockers} [21,22]. Earlier studies also indicated that AngII stimulated both myocardin expression and target gene transcription in VSMCs (vascular smooth muscle cells) to cause VSMC hypertrophy [23,24]. AngII has also been identified as a stimulator of cardiomyocyte hypertrophy in many studies [25,26]. However, the expression of AngII and myocardin after hypoxia and their relationship in regulating gene transcription in cardiomyocytes in response to hypoxia has not been well defined. Therefore we developed the hypothesis that hypoxia-related myocardial injury may result in cardiomyocyte hypertrophy by expressing certain cardiac-restricted genes through specific signal transductional pathways. Myocardin may play an important role in regulating this expression. Hypertrophic markers (ANF and BNP) and cardiomyocyte-restricted gene modulation may also be identified in response to myocardin expression through specific signal transduction pathways after hypoxia. AngII, a potent stimulator of cardiomyocyte hypertrophy, may be expressed in response to hypoxia to enhance myocardin expression and cardiomyocyte gene transcription as shown previously in VSMC models [23,24]. However, the specific transduction pathway(s) mediating these mechanisms remain to be identified after hypoxic injury.
In the present study, we investigated the effect of hypoxia on the expression of myocardin in neonatal cardiomyocytes and the potential signalling mechanisms mediating its expression. We demonstrate that hypoxia in neonatal cardiomyocytes increased the expression of myocardin and this appeared to be mediated via AngII and the ERK pathway. In addition, cardiomyocytes exposed to hypoxia had an increase in the expression of BNP and MHC (myosin heavy chain), increased [3H]proline incorporation in cardiomyocytes, and increased transcriptional activities inducing cardiomyocyte hypertrophy.
MATERIALS AND METHODS
Primary culture of left ventricular cardiomyocytes
Cardiomyocytes were obtained from 2–3-day-old Wistar rats by trypsinization, as described previously [27,28]. Cultured myocytes thus obtained were >95% pure, as revealed by observing their contractile characteristics using a light microscope and staining with an anti-desmin antibody (Dako Cytomation). The culture medium consisted of DMEM/F12 (Dulbecco's modified Eagle's medium/Ham's F12) supplemented with 20% (v/v) knockout serum replacement, 1% (v/v) non-essential amino acids, 2 mmol/l L-glutamine, 0.1% 2-mercaptoethanol and 0.1% of a commercially available penicillin/streptomycin stock solution (100 units/ml penicillin and 100 μg/ml streptomycin; Invitrogen). Cells were then transferred to serum-free medium and maintained for another 2 days. The enriched myocytes were then subjected to hypoxia.
The animals were handled according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No 85-23, revised 1996). The study was reviewed and approved by the Institutional Animal Care and Use Committee of Shin Kong Wu Ho-Su Memorial Hospital.
Induction of hypoxia
Hypoxic conditions were achieved by adding medium pre-equilibrated with nitrogen gas to cells prior to the incubation in a Plexiglas chamber purged with water-saturated nitrogen gas using an oxygen controller (PROOX model 110; BioSpherix). The PO2 (partial pressure of oxygen) of the culture medium under hypoxia was monitored using an ISO2 dissolved oxygen meter (World Precision Instruments). Measurements indicated that a steady state in the culture medium was maintained during the experiments. Hypoxic culture medium (BioSpherix C-chamber) was used with mixed air in and out controlled by a BioSpherix PROOX incubator. Hypoxia settings were: (i) 10% O2, 5% CO2 and 85% N2; (ii) 5% O2, 5% CO2 and 90% N2; and (iii) 2.5% O2, 5% CO2 and 92.5% N2. A lower oxygen concentration (1%) was attempted, but cardiomyocytes had difficulty surviving under these conditions. The duration of hypoxia was 1 h, 2 h, 4 h, 6 h or longer. The content of the culture was left unchanged during the experiment.
Antibodies and reagents
A rabbit polyclonal antibody against myocardin, mouse mAbs (monoclonal antibodies) against JNK (c-Jun N-terminal kinase) and an anti-actin antibody were obtained from Santa Cruz Biotechnology. Mouse mAbs against p38 MAPK (mitogen-activated protein kinase), ERK (extracellular-signal-regulated kinase), phospho-ERK and PI3K (phosphoinositide 3-kinase) were purchased from BD Bioscience Pharmingen. PD98059, SB203580, SP600125 and wortmannin were purchased from Calbiochem. All other chemicals of reagent grade were obtained from Sigma. The roles of JNK, p38 MAPK, ERK and PI3K in hypoxia-induced myocardin expression were determined by pre-treatment of the myocytes with 25 μmol/l SP600125 (a potent cell-permeant selective and reversible inhibitor of JNK), 3 μmol/l SB203580 (a highly specific cell-permeant inhibitor of p38 MAPK), 50 μmol/l PD98059 (a specific and potent inhibitor of the ERK pathway) or 50 nM wortmannin (a specific and potent inhibitor of PI3K) for 30 min before hypoxia. AngII and the anti-AngII antibodies were from Bachem. To examine the effect of ARBs, cardiomyocytes were treated with 100 nmol/l losartan (Merck).
RNA isolation and real-time quantitative PCR
Total cellular RNA was extracted from cardiomyocytes (approx. 1.5×106 cells) using TRI reagent (Molecular Research Center, Cincinnati, OH, U.S.A.), according to the manufacturer's instructions. A total of 1 μg of total RNA was reverse-transcribed by M-MuLV reverse transcriptase (Finnzyme) in a total volume of 20 μl. The reverse transcriptase products were amplified with DyNAmo HS SYBR Green qPCR Kit (Finnzyme) in the reaction mix containing DyNAmo SYBR Green master mix and primers. Primers were designed for detection of myocardin gene expression (forward: 5′-GGACTGCTCTGGCAACCCAGTGC-3′; reverse: 5′-CATCTGCTGACTCCGGGTCATTTGC-3′). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene expression was used as internal controls (forward: 5′-GAGAGGCTCTCTGTCGACTAC-3′; reverse: 5′-TAGTGTAGGTTGGGCGCTCAA-3′) and did not change under the above conditions.
Western blot analysis
Cardiomyocytes were washed in PBS and lysed with RIPA buffer containing 1% (v/v) Nonidet P40, 0.5% SDS and protease inhibitor cocktail containing 10 μg/ml PMSF, 2 μg/ml leupeptin, 2 μg/ml pepstatin A and 2 μg/ml aprotinin. Cells were disrupted by intermittent sonication. After centrifugation, the amount of protein in the supernatant was measured using BSA as a standard. Cell lysates were then subjected to SDS/PAGE followed by Western blotting. Antigen–antibody complexes were detected by HRP (horseradish peroxidase)-labelled rabbit anti-(mouse IgG) or goat anti-(rabbit IgG) with an ECL (enhanced chemiluminescence) detection system (Pierce).
RNA interference
Neonatal cardiomyocytes were transfected with 800 ng of ERK- and myocardin-annealed siRNA (small interfering RNA; Dharmacon). ERK siRNAs are target-specific 20–25-nt siRNAs designed to knock down gene expression. siRNA sequences were 5′-GACCGGAUGUUAACCUUUAUU-3′ (sense) and 5′-PUAAAGGUUAACAUCCGGUCUU-3′ (antisense) for ERK. Myocardin siRNA sequences were 5′-UGCAACUGCAGAAGCAGAAUU-3′ (sense) and 5′-UGCAACUGGUCUUGCAGAAUU-3′ (scramble). As a negative control, a non-targeting (control) siRNA (Dharmacon) was used. For transfection of neonatal cardiomyocytes with siRNA oligonucleotides, Effectene transfection reagent was used according to the manufacturer's instructions (Qiagen). After incubation at 37 °C, cells were kept under hypoxia and analysed by Western blotting.
EMSA (electophoretic mobility-shift assay)
Nuclear protein concentrations from cultured myocytes were determined using the Bradford method (Bio-Rad Laboratories). Consensus and control oligonucleotides (Research Biolabs) were labelled by polynucleotide kinase incorporation of [γ-32P]ATP. The consensus oligonucleotide sequence of myocardin was 5′-GGACTGCTCTGGCAACCCAGTGC-3′ and the reverse primer was 5′-CATCTGCTGACTCCGGGTCATTTGC-3′. The myocardin mutant oligonucleotide sequence was 5′-GGACTGCTCTTTCAACCCAGTGC-3′. EMSA was performed as described previously [24]. In each case, mutant or unlabelled oligonucleotide was used as a control to compete with labelled sequences.
ELISA for AngII
AngII was measured in cell lysates and the culture medium by a quantitative competitive ELISA, using a specific anti-AngII antibody (Peninsula Labs), as described previously [29].
Promoter activity assay
A −968 to +44 bp rat myocardin promoter construct was generated as follows. Rat genomic DNA was amplified with forward (5′-GGACTGCTCTGGCAACCCAGTGC-3′) and reverse (5′-CATCTGCTGACTCCGGGTCATTTGC-3′) primers. The amplified product was digested with MluI and BglII restriction enzymes and ligated into the pGL3-basic luciferase plasmid vector (Promega) digested with the same enzymes. The myocardin promoter contains myocardin-conserved sites (GGGACTT) at −285 to −279 bp. For the mutant, the myocardin-binding sites were mutated using a mutagenesis kit (Stratagene). Site-specific mutations were confirmed by DNA sequencing. Plasmids were transfected into cardiomyocytes using a low-pressure accelerated gene gun (Bioware).
Determination of protein synthesis
Protein synthesis was examined by measuring [3H]proline incorporation into the cells. Cultured cardiomyocytes were divided into the following groups: (i) control group, in which the cells were cultured in serum-free DMEM/F12; and (ii) hypoxia group, in which cells were cultured in 2.5% O2 in serum-free DMEM/F12. Each experiment was repeated six times. Cardiomyocytes were first grown in DMEM/F12 with 10% (v/v) FBS (fetal bovine serum) and 200 mg/l L-glutamine, and then seeded in 24-well plates at 1×105 cells/well in DMEM/F12+10% (v/v) FBS. After synchronization of cardiomyocytes, the medium was changed to serum-free DMEM/F12. Cardiomyocytes were incubated under hypoxia and exposed to [3H]proline (1 μCi/well) for the last 8 h of the 24 h incubation period. After incubation, cells were washed with ice-cold PBS and 10% (v/v) TCA (trichloroacetic acid)-insoluble [3H]proline was collected on glass fibre filters (Whatman) and the radioactivity was determined using a liquid-scintillation counter (LS 6500; Beckman).
Statistical analysis
All results were expressed as means±S.D. Statistical significance was evaluated using ANOVA, followed by a Tukey–Kramer multiple comparisons test (GraphPad Software). A value of P<0.05 was considered as statistically significant.
RESULTS
Hypoxia increases the expression of myocardin in cultured cardiomyocytes
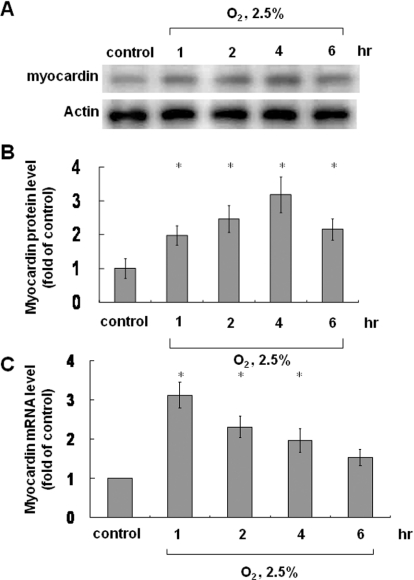
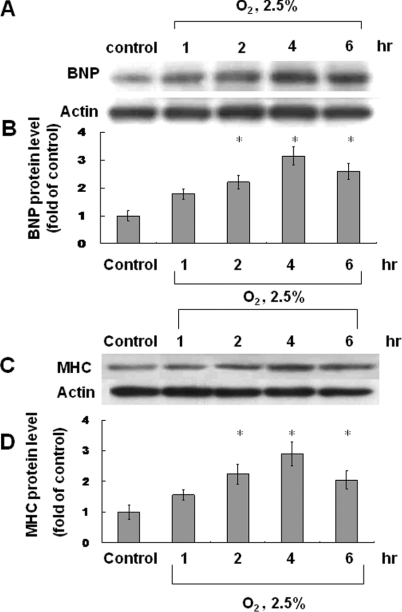
We used different degrees of hypoxia (10%, 5% and 2.5% O2) for various times (1, 2, 4 and 6 h). Myocardin expression was most evident in cells cultured in 2.5% O2 for 4 h compared with the other conditions (Supplementary Figure S1 at http://www.clinsci.org/cs/119/cs1190273add.htm) and, therefore, we used this concentration for further analysis. There was no significant difference in pH between the different degrees of hypoxia. In 2.5% O2, myocardin mRNA was maximally expressed after 1 h and decreased gradually thereafter (Figure 1C). Myocardin protein levels also increased gradually and reached a peak after 4 h (Figures 1A and 1B). Protein levels were suppressed using myocardin siRNA (Supplementary Figure S2 at http://www.clinsci.org/cs/119/cs1190273add.htm).
Figure 1. Effect of hypoxia on myocardin protein and mRNA levels.
Neonatal cardiomyocytes were subjected to hypoxia for 1–6 h, and total cell lysates were immunoblotted with an anti-myocardin antibody (A) or (C) extracted to determine mRNA levels. (A and B) Myocardin protein level increased and reached a peak after 4 h of hypoxia. Actin is used to show equal amounts of protein loading in each lane. (C) Myocardin mRNA levels reached a peak after 1 h of hypoxia and then declined. *P<0.01 compared with normoxia control (n=3).
Hypoxia increases the expression of myocardin in cultured cardiomyocytes through the ERK pathway
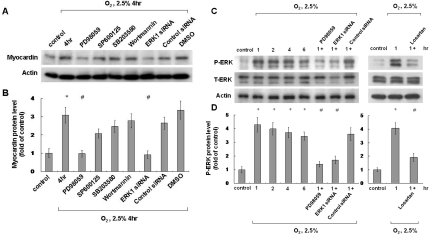
Previous studies have indicated that hypoxia may stimulate gene expression through different pathways, including ERK, JNK, p38 MAPK and PI3K/Akt [30], and therefore hypoxia may increase the expression of myocardin through one of these pathways. Different signalling pathway inhibitors were used to investigate which pathway was involved (PD98059, an ERK pathway inhibitor, SP600125, a JNK inhibitor, SB203580, a p38 MAPK inhibitor, and wortmannin, a PI3K inhibitor). Of the inhibitors used, myocardin expression was blocked most effectively by PD98059, and similar effects were also achieved using ERK siRNA (Figures 2A and 2B).
Figure 2. Effect of signalling pathway inhibitors on hypoxia-induced myocardin expression and ERK phosphorylation.
(A and B) ERK pathway mediates hypoxia-induced myocardin expression in neonatal cardiomyocytes. Neonatal cardiomyocytes were pre-treated with an ERK pathway inhibitor (PD98059), a JNK inhibitor (SP600125), a p38 MAPK inhibitor (SB203580), a PI3K/Akt inhibitor (wortmannin) or ERK siRNA, followed by hypoxia for 4 h. Neonatal cardiomyocytes were harvested and cell lysates were analysed by Western blotting using an anti-myocardin antibody. Result are normalized to actin levels. *P<0.01 compared with normoxia control; #P<0.01 compared with hypoxia for 4 h (n=3). (C and D) Hypoxia-induced phosphorylation of ERK in neonatal cardiomyocytes. Neonatal cardiomyocytes were subjected to normoxia or hypoxia for 1–6 h in the presence or absence of inhibitors, and cell lysates were collected for Western blot analysis using antibodies against total ERK (T-ERK) and phospho-ERK (P-ERK). *P<0.01 compared with normoxia control; #P<0.01 compared with hypoxia for 1 h (n=3).
Hypoxia increases ERK phosphorylation in cultured neonatal cardiomyocytes
ERK phosphorylation was also increased to a maximal level 1 h after 2.5% O2 and then declined gradually thereafter (Figures 2C and 2D). PD98059 and ERK siRNA were effective in blocking ERK phosphorylation induced by 2.5% O2 (Figures 2C and 2D). In addition, ERK phosphorylation was also suppressed by losartan (Figures 2C and 2D).
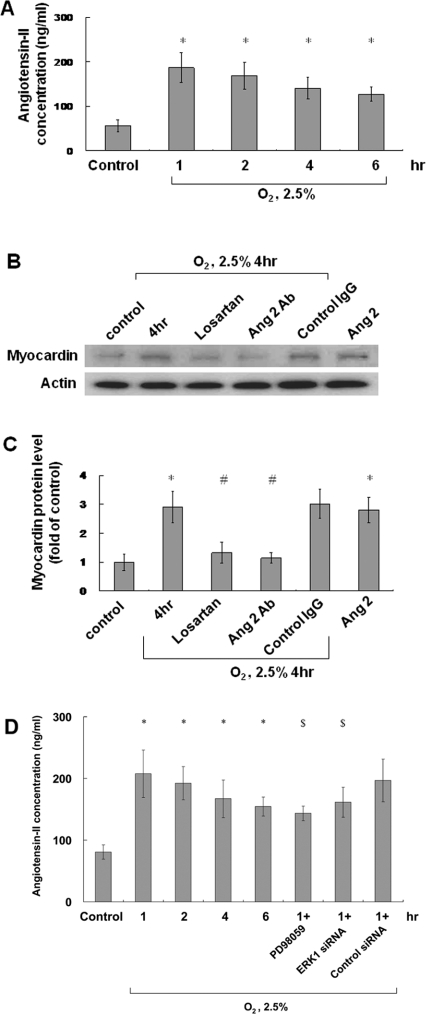
AngII induced by hypoxia increases myocardin levels in cultured cardiomyocytes
Under 2.5% O2, AngII protein levels increased and reached a peak 1 h later (earlier than the maximum expression of myocardin after 4 h) before declining gradually thereafter (Figure 3A). Exogenous addition of AngII (10 nmol/l) increased myocardin protein expression similar to 2.5% O2 (Figure 3C). Myocardin protein levels increased after hypoxia, but were suppressed by losartan (100 nmol/l) and the anti-AngII antibody (Figures 3B and 3C). Hypoxia-induced AngII expression was partially suppressed by PD98059 and ERK siRNA (Figure 3D).
Figure 3. Hypoxia induces AngII expression and mediates hypoxia-induced myocardin expression.
(A) AngII was measured in cell lysates and the culture medium by a quantitative competitive ELISA, using a specific anti-AngII antibody, following 1–6 h of hypoxia. (B and C) Neonatal cardiomyocytes were subjected to normoxia or hypoxia for 4 h in the absence or presence of losartan (100 nmol/l) or an anti-AngII antibody and the addition of exogenous AngII (10 nmol/l). Total cell lysates were immunoblotted with an anti-myocardin antibody. Actin is used to show equal amounts of protein loading in each lane. (D) Hypoxia-induced AngII expression was suppressed by PD98059 and ERK siRNA. *P<0.01 compared with normoxia control; #P<0.01 compared with 4 h of hypoxia; $P<0.05 compared with 1 h of hypoxia (n=3).
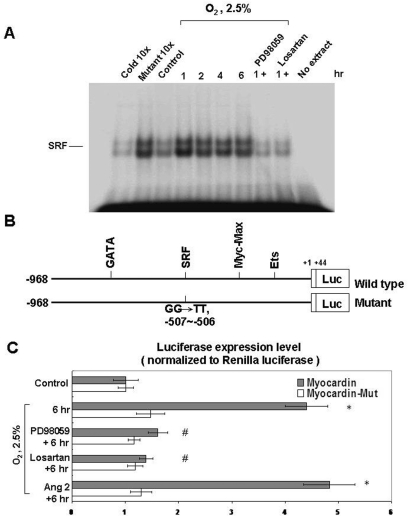
Hypoxia increases the binding of myocardin and SRF, and enhances the gene transcription activity of myocardin
Under 2.5% O2, increased binding activity between myocardin and SRF was detected by EMSA, and PD98059 and losartan decreased this binding activity (Figure 4A). This finding indicated that hypoxia in cardiomyocytes may increase the co-operative binding between myocardin and SRF. Furthermore, hypoxia-induced AngII expression and ERK phosphorylation may increase myocardin expression and myocardin/SRF binding activity.
Figure 4. Hypoxia induces myocardin/SFR binding (A) and myocardin transcriptional activity (B and C) in cardiomyocytes.
(A) Increased binding between myocardin and SRF under 2.5% O2 as determined using an EMSA. The binding between myocardin and SRF was suppressed by PD98059 and losartan. (B) Wild-type and myocardin mutant used for the luciferase assay. (C) Hypoxia (2.5% O2) increased the transcriptional activity of myocardin when compared with the myocardin mutant (Mut) as determined using a luciferase reporter assay. The transcriptional activity was suppressed by PD98059 and losartan. Addition of exogenous AngII (10 nmol/l) increased the transcriptional activity in neonatal cardiomyocyte similar to that of 2.5% O2. *P<0.01 compared with normoxia control; #P<0.01 compared with 6 h (n=3).
We also used a luciferase reporter assay to identify the gene transcription activity of myocardin in cardiomyocytes under hypoxia (Figure 4B). We found that hypoxia increased transcriptional activity of myocardin, whereas a myocardin mutant failed to have the same effect. Exogenous addition of AngII also increased the transcriptional activity of myocardin. PD98508 and losartan suppressed the transcriptional activity of myocardin.
Hypoxia in cardiomyocytes increases myocardin expression in the nucleus, hypertrophic marker and phenotype modulation, and gene transcription to induce cardiomyocyte hypertrophy
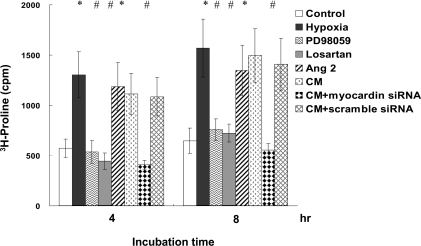
In cardiomyocytes under hypoxia, we have shown increased myocardin expression and enhanced transcriptional activity in cardiomyocytes. Next, we used confocal microscopy to identify the effect of myocardin on cardiomyocytes (Supplementary Figure S3 at http://www.clinsci.org/cs/119/cs1190273add.htm). Confocal microscopy showed increased myocardin expression in the nucleus from 2 to 4 h after hypoxia, which contributed to a hypertrophic change in the cardiomyocytes (Supplementary Figures 3B and 3C). AngII increased further the expression of myocardin in the nucleus of cardiomyocytes (Supplementary Figure 3F), and myocardin expression was suppressed by PD98059 (Supplementary Figure 3D) and losartan (Supplementary Figure 3E). The effect on BNP, a hypertrophic cardiomyocyte marker, was also determined after hypoxia treatment. Results showed that BNP protein levels increased after hypoxia and reached a peak after 4 h (Figures 5A and 5B). MHC, a major cardiomyocyte phenotype marker, also increased significantly after hypoxia-induced cardiomyocyte hypertrophy (Figure 5C and 5D). We also evaluated protein synthesis in cardiomyocytes by measuring [3H]proline incorporation into the cells. Results showed an increase in protein synthesis after hypoxia for 4 to 8 h, which represented a hypertrophic change in the cardiomyocytes (Figure 6). Pre-treatment with PD98059, losartan and myocardin siRNA inhibited the hypoxia-induced protein synthesis. The exogenous addition of AngII and conditioned medium also increased [3H]proline incorporation similar to the effect of hypoxia.
Figure 5. Hypoxia induces the expression of BNP and MHC in cardiomyocytes.
BNP (A and B) and MHC (C and D) protein levels were both increased after hypoxia (2.5% O2), reaching peak levels after 4 h.*P<0.01 compared with normoxia control (n=3).
Figure 6. Hypoxia induces protein synthesis in neonatal cardiomyocytes.
Incorporation of [3H]proline into neonatal cardiomyocytes increased after hypoxia and the exogenous addition of AngII for 4–8 h, and was suppressed by PD98059, losartan, and myocardin siRNA. *P<0.01 compared with normoxia control; #P<0.01 compared with hypoxia (n=6). CM, conditioned medium.
DISCUSSION
Hypoxic/ischaemic injury is a commonly observed pathological phenomenon in cardiovascular diseases, including coronary artery disease, carotid stenosis, myocardial infarction and heart failure. In cardiomyocytes, hypoxic/ischaemic injury forms a stress phenomenon resulting in the expression of fetal cardiac-restricted genes [1–4]. Cardiomyocyte hypertrophy is the first adaptive phenomenon, with enlargement of cells and organelles, and may finally result in heart failure or dilated cardiomyopathy, which remains a major contributor to cardiovascular mortality and morbidity [4,5]. In the present study, the following novel findings are reported: (i) the effect of hypoxia on myocardin to cause cardiomyocyte hypertrophy; (ii) the effect of hypoxia on the expression of AngII, and the effect of AngII on the induction of myocardin expression and gene transcription in neonatal cardiomyocytes to cause hypertrophy; and (iii) the specific signal transduction pathway mediating myocardin expression under hypoxia.
The induction of AngII in cardiac myocytes to cause cardiomyocyte hypertrophy may be controversial. Recent studies have indicated that hypoxia in cardiomyocytes results in cardiomyocyte hypertrophy and remodelling, and could be inhibited by ARBs [21,22]. Some studies have also indicated that AngII expression after different mechanical loads may induce cardiomyocyte hypertrophy through different signal transduction pathways [25,26]. However, Zou et al. [31] have shown that mechanical stress activates the AT1R without the involvement of AngII and via an AngII-independent mechanism in cardiomyocytes. Without the involvement of AngII, mechanical stress not only activates ERK and increases phosphoinositide production in vitro, but also induces cardiac hypertrophy in vivo. Mechanical stretch also induces the association of the AT1R with JAK2 (Janus kinase 2) and the translocation of G-proteins into the cytosol.
The effect of hypoxia on the expression of AngII and the induction of cardiomyocyte hypertrophy by AngII after hypoxia have not been studied before. In the present study, AngII protein levels increased and reached a peak 1 h after hypoxia, which indicated that hypoxic injury also stimulated AngII expression. Our present study also indicated that both hypoxia-induced AngII expression and exogenous addition of AngII (without hypoxia) increased transcriptional activity in neonatal cardiomyocytes to cause cardiomyocyte hypertrophy. Our present results suggest that: (i) hypoxic injury to cardiomyocytes also stimulates the expression of AngII, similar to other mechanical loads; and (ii) hypoxic-induced AngII expression plays a role in cardiomyocyte hypertrophy through increased gene transcription.
Furthermore, previous studies have not identified the relationship between AngII and myocardin and their regulation by gene transcription in cardiomyocytes. Earlier studies indicated that AngII-induced smooth muscle α-actin gene transcription would result in VSMC hypertrophy [23]. That study indicated further that myocardin stimulation and VSMC gene transcription was enhanced by AngII through AT1Rs [23]. CArG boxes in VSMCs are necessary for AngII-induced gene transcription. Both dominant-negative myocardin and myocardin siRNA decreased AngII-mediated VSMC gene transcription. Therefore the co-operative effect of AngII and myocardin increased gene transcription in VSMCs to induce VSMC hypertrophy [23]. However, no other previous studies have discussed the relationship between AngII and myocardin under hypoxia to cause cardiomyocyte hypertrophy. Our present study indicated that hypoxia-induced AngII expression stimulated both myocardin expression and myocardin-related gene transcription in neonatal cardiomyocytes. In addition, our present study has also identified the signal transductional pathway responsible for mediating the expression of myocardin under hypoxia. By using signal transduction pathway inhibitors, the ERK pathway was shown to be responsible for the induction of myocardin. Similarly, ERK phosphorylation enhanced the expression of myocardin, and ERK siRNA repressed the expression of myocardin. Thus we can conclude that the ERK pathway mediates the induction of myocardin expression after hypoxia.
In regard to the relationship between AngII and ERK, previous studies [26,32] and our present study have indicated that AngII expression may be induced via the ERK pathway to cause cardiomyocyte hypertrophy. Thus the ARB could effectively suppress ERK phosphorylation by blocking the expression of AngII to prevent cardiac hypertrophy (Figures 2C and 2D). Hypoxia-induced AngII expression was a novel finding in the present study. We found that the ERK pathway inhibitor PD98059 and/or ERK siRNA could inhibit the expression of AngII, but the effect was partial (Figure 3D). This finding suggests that hypoxia-induced AngII expression may possibly be activated by several different pathways and that the ERK pathway is one of these signalling pathways.
With regards to myocardin-related cardiomyocyte hypertrophy, previous studies have also investigated this [14–17,33]. Some pro-hypertrophic agonists, such as AngII or ET-1 (endothelin-1), can result in cardiomyocyte hypertrophy by increasing transcriptional activities in cardiomyocytes. Multiple signal transductional cascades may be involved in these hypertrophic mechanisms. According to our present results, we identified ERK to be involved in the signal transduction pathway facilitating AngII-stimulated myocardin-related gene transcription during hypoxia. Myocardin-related gene transcription in cardiomyocytes is through myocardin/SRF binding to the CArG boxes of target genes. We also found that AngII- and ERK-mediated cardiomyocyte hypertrophy after hypoxia could be induced by myocardin as not only the ERK pathway inhibitor and ARB, but also myocardin siRNA inhibited protein synthesis (representing cardiomyocyte hypertrophy) in neonatal cardiomyocytes under hypoxia. Another signal pathway not related to myocardin which causes cardiomyocyte hypertrophy involves the binding of the transcriptional factor GATA4 to GATA4 sites of target genes [20]. A previous study has identified GSK (glycogen synthase kinase) 3β, a ubiquitously expressed serine/threonine kinase, as having a role in inhibiting myocardin expression through the PI3K/Akt pathway [20]. Furthermore, that study, performed in cultured cardiomyocytes and transgenic mice, has shown that GSK3β inactivation by phosphorylation is necessary and sufficient for the development of cardiomyocyte hypertrophy [20]. Our present study found that an ARB is another potent agent capable of inhibiting gene transcription and cardiomyocyte hypertrophy by suppressing AngII and myocardin simultaneously through the ERK pathway. Therefore commercially available ARBs may be suitable for treating patients with myocardial hypertrophy, hypertrophic cardiomyopathy and hypertension to prevent further cardiomyocyte hypertrophy and heart failure.
In conclusion, hypoxia in cultured rat neonatal cardiomyocytes induces the expression of AngII. AngII and the ERK pathway mediate the induction of myocardin expression, with subsequent cardiomyocyte hypertrophy through increased transcriptional activities. Cardiomyocyte hypertrophy after hypoxia could be identified by the expression of the hypertrophic marker (BNP) and the cardiomyocyte phenotype marker (MHC), increased protein synthesis, and hypertrophic changes in cardiomyocytes. Hypoxia-induced AngII expression has a similar effect to the exogenous addition of AngII and may increase both the expression of myocardin and transcriptional activity in cardiomyocytes. Cardiomyocyte hypertrophy can be prevented using an ARB, which inhibits the effect of AngII and myocardin.
Online data
FUNDING
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1.Miano J. M. Serum response factor: toggling between disparate programs of gene expression. J. Mol. Cell. Cardiol. 2003;35:577–593. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- 2.Wang D., Chang P. S., Wang Z., Sutherland L., Richardson J. A., Small E., Krieg P. A., Olson E. N. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X., Azhar G., Zhong Y., Wei J. Y. Identification of a novel serum response factor cofactor in cardiac gene regulation. J. Biol. Chem. 2004;279:55626–55632. doi: 10.1074/jbc.M405945200. [DOI] [PubMed] [Google Scholar]
- 4.Wang D. Z., Li S., Hockemeyer D., Sutherland L., Wang Z., Schratt G. Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc. Natl. Acad. Sci. U.S.A. 2002;99:14855–14860. doi: 10.1073/pnas.222561499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kontaraji J. E., Parthenakis F. I., Patrianakos A. P., Karalis I. K., Vardas P. E. Altered expression of early marker genes in circulating cells of patients with hypertrophic cardiomyopathy. Cardiovasc. Pathol. 2007;16:329–335. doi: 10.1016/j.carpath.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Deindl E. Arteriogenesis: a focus on signal transduction cascades and transcription factors. Thromb. Haemostasis. 2007;98:940–943. [PubMed] [Google Scholar]
- 7.Wen J. K., Han M. Myocardin, a novel potentiator of SRF-mediated transcription in cardiac muscle. Mol. Cell. 2001;8:1–2. doi: 10.1016/s1097-2765(01)00297-0. [DOI] [PubMed] [Google Scholar]
- 8.Tuyn J., Knan-Shanzer S., Watering M. J. M., Graaf M., Laarse A., Schalij M. J., van der Wall E. E., de Vries A. A., Atsma E. E. Activation of cardiac and smooth muscle-specific genes in primary human cells after forced expression of human myocardin. Cardiovasc. Res. 2005;67:245–255. doi: 10.1016/j.cardiores.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Firulli A. B. Another hat for myocardin. J. Mol. Cell. Cardiol. 2002;34:1293–1296. doi: 10.1006/jmcc.2002.2098. [DOI] [PubMed] [Google Scholar]
- 10.Teg Pipes G. C., Creemers E. E., Olson E. N. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 2006;20:1545–1556. doi: 10.1101/gad.1428006. [DOI] [PubMed] [Google Scholar]
- 11.Parmacek M. S. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ. Res. 2007;100:633–644. doi: 10.1161/01.RES.0000259563.61091.e8. [DOI] [PubMed] [Google Scholar]
- 12.Kuwahara K., Barrientos T., Teg Pipes G. C., Li S., Olson E. N. Muscle-specific signaling mechanism that links actin dynamics to serum response factor. Mol. Cell. Biol. 2005;25:3173–3181. doi: 10.1128/MCB.25.8.3173-3181.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellstrand P., Albinson S. Stretch-dependent growth and differentiation in vascular smooth muscle: role of the actin cytoskeleton. Can. J. Physiol. Pharmacol. 2005;83:869–875. doi: 10.1139/y05-061. [DOI] [PubMed] [Google Scholar]
- 14.Konhilas J. P., Leinwand L. A. Partnering up for cardiac hypertrophy. Circ. Res. 2006;98:985–987. doi: 10.1161/01.RES.0000221823.31424.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pijnappels D. A., Tuyn J., Vries A. F., Grauss R. W., Laarse A., Ypey D. L., Atsma D. E., Schalij M. J. Resynchronization of separated rat cardiomyocyte fields with genetically modified human ventricular scar fibroblasts. Circulation. 2007;116:2018–2028. doi: 10.1161/CIRCULATIONAHA.107.712935. [DOI] [PubMed] [Google Scholar]
- 16.Tuyn J., Pijnappels D. A., Vries A. F., Vries I., Dijke I. V., Knan-Shanzer S., van der Laarse A., Schalij M. J., Atsma D. E. Fibroblasts from human postmyocardial infarction scars acquire properties of cardiomyocytes after transduction with a recombinant myocardin gene. FASEB J. 2007;21:3369–3379. doi: 10.1096/fj.07-8211com. [DOI] [PubMed] [Google Scholar]
- 17.Xing W., Zhang T. C., Cao D., Wang Z., Antos C. L., Li S., Wang Y., Olson E. N., Wang D. Z. Myocardin induces cardiomyocyte hypertrophy. Circ. Res. 2006;98:1089–1097. doi: 10.1161/01.RES.0000218781.23144.3e. [DOI] [PubMed] [Google Scholar]
- 18.Torrado M., Lopez E., Centeno A., Medrano C., Castro-Beiras A., Mikhailov A. T. Myocardin mRNA is augmented in the failing myocardium: expression profiling in the porcine model and human dilated cardiomyopathy. J. Mol. Med. 2003;81:566–577. doi: 10.1007/s00109-003-0470-7. [DOI] [PubMed] [Google Scholar]
- 19.Miano J. M. Channeling to myocardin. Circ. Res. 2004;95:340–342. doi: 10.1161/01.RES.0000140893.16465.2d. [DOI] [PubMed] [Google Scholar]
- 20.Badorff C., Seeger F. H., Zeiher A. M., Dimmeler S. Glycogen synthase kinase 3β inhibits myocardindependent transcription and hypertrophy induction through site-specific phosphorylation. Circ. Res. 2005;97:645–654. doi: 10.1161/01.RES.0000184684.88750.FE. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita C., Hayashi T., Mori T., Tazawa N., Kwak C. J., Nakano D., Sohmiya K., Okada Y., Kitaura Y., Matsumura Y. Angiotensin II receptor blocker reduces oxidative stress and attenuates hypoxia-induced left ventricular remodeling in apolipoprotein E-knockout mice. Hypertens. Res. 2007;30:1219–1230. doi: 10.1291/hypres.30.1219. [DOI] [PubMed] [Google Scholar]
- 22.Inamoto S., Hayashi T., Tazawa N., Mori T., Yamashita C., Nakano D., Sohmiya K., Okada Y., Kitaura Y., Matsumura Y. Angiotensin-II receptor blocker exerts cardioprotection in diabetic rats exposed to hypoxia. Circ. J. 2006;70:787–792. doi: 10.1253/circj.70.787. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida T., Hoofnagle M. H., Owens G. K. Myocardin and Prx1 contribute to angiotensin II-induced expression of smooth muscle α-actin. Circ. Res. 2004;94:1075–1082. doi: 10.1161/01.RES.0000125622.46280.95. [DOI] [PubMed] [Google Scholar]
- 24.Chang H., Shyu K. G., Wang B. W., Kuan P. Regulation of hypoxia inducible factor 1-α by cyclical mechanical stretch in rat vascular smooth muscle cells. Clin. Sci. 2003;105:447–456. doi: 10.1042/CS20030088. [DOI] [PubMed] [Google Scholar]
- 25.Sadoshima J., Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu. Rev. Physiol. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- 26.Sadoshima J., Malhotra R., Izumo S. The role of the cardiac renin-angiotensin system in load-induced cardiac hypertrophy. J. Card. Failure. 1996;2:S1–S6. doi: 10.1016/s1071-9164(96)80052-2. [DOI] [PubMed] [Google Scholar]
- 27.Shyu K. G., Chen C. C., Wang B. W., Kuan P. Angiotensin II receptor antagonist blocks the expression of connexin43 induced by cyclical mechanical stretch in cultured neonatal rat cardiac myocytes. J. Mol. Cell. Cardiol. 2001;33:691–698. doi: 10.1006/jmcc.2000.1333. [DOI] [PubMed] [Google Scholar]
- 28.Hannan R. D., Luyken J., Rothblum L. I. Regulation of ribosomal DNA transcription during contraction-induced hypertrophy of neonatal cardiomyocytes. J. Biol. Chem. 1996;271:3213–3220. doi: 10.1074/jbc.271.6.3213. [DOI] [PubMed] [Google Scholar]
- 29.Baker K. M., Chernin M. I., Schreiber T., Sanghi S., Haiderzaidi S., Booz G. W., Dostal D. E., Kumar R. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul. Pept. 2004;120:5–13. doi: 10.1016/j.regpep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Seta K. A., Millhorn D. E. Functional genomics approach to hypoxia signaling. J. Appl. Physiol. 2004;96:765–773. doi: 10.1152/japplphysiol.00836.2003. [DOI] [PubMed] [Google Scholar]
- 31.Zou Y., Akazawa H., Qin Y., Sano M., Takano H., Minanino T., Makita N., Iwanaqa K., Zhu W., Kudoh S., et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat. Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki T., Komuro I., Yazaki Y. Role of renin–angiotensin system in cardiac hypertrophy. Am. J. Cardiol. 1999;83:53–57. doi: 10.1016/s0002-9149(99)00259-3. [DOI] [PubMed] [Google Scholar]
- 33.Kontaraki J. E., Parthenakis F. I., Patrianakos A. P., Karalis I. K., Vardas P. E. Myocardin gene regulatory variants as surrogate markers of cardiac hypertrophy: study in a genetically homogeneous population. Clin. Genet. 2008;73:71–78. doi: 10.1111/j.1399-0004.2007.00932.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.